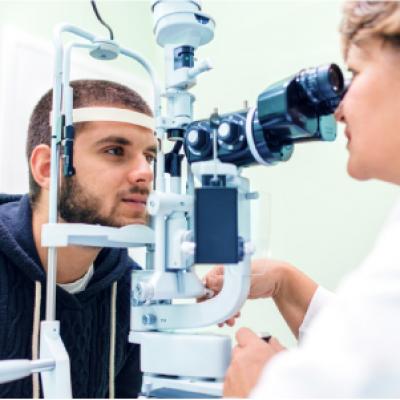
Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR) represent an extraordinary breakthrough in regenerative medicine, offering a promising solution to one of the most debilitating complications of diabetes mellitus. Diabetic Retinopathy, the leading cause of vision loss in working-age adults worldwide, results from chronic hyperglycemia-induced damage to the retinal microvasculature. As the condition progresses, patients experience hemorrhages, microaneurysms, macular edema, retinal ischemia, and ultimately, neovascularization leading to irreversible blindness.
Traditional management options, including glycemic control, anti-VEGF injections, laser photocoagulation, and vitrectomy, only target the symptoms or slow the progression—none can regenerate the retinal tissue. In contrast, Cellular Therapy and Stem Cells for Diabetic Retinopathy aim to restore retinal structure and function by targeting the root pathology. This comprehensive introduction explores the potential of stem cell-based therapeutics to repair damaged retinal vasculature, restore visual function, and prevent disease advancement. It also highlights the cutting-edge strategies developed at the Anti-Aging and Regenerative Medicine Center of Thailand by DrStemCellsThailand (DRSCT), where innovation meets clinical excellence to combat retinal blindness through personalized cellular therapy [1-4].
Rethinking DR Management: Why Conventional Therapies Fall Short
Despite advancements in ophthalmology, current treatment protocols for Diabetic Retinopathy remain palliative, focusing largely on halting disease progression. These therapies fail to reverse neurovascular degeneration or replenish the lost retinal cells. Laser therapies cause retinal scarring; intravitreal anti-VEGF injections must be repeated indefinitely and lose efficacy in advanced stages; and surgical interventions are invasive with limited visual restoration.
What is needed is a therapeutic paradigm that doesn’t merely manage—but regenerates. Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR) fulfill this demand by addressing neurovascular injury, oxidative damage, and inflammatory imbalance directly at the cellular and molecular levels. Stem cell-based approaches open the possibility of not just slowing blindness but actually reversing the degenerative process—turning hope into a tangible future [1-4].
2. The Science Behind the Promise: Cellular Therapy and Stem Cells for Diabetic Retinopathy
Imagine a world where patients at risk of blindness can regain vision through minimally invasive regenerative protocols. Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR) strive toward this future. Mesenchymal stem cells (MSCs), endothelial progenitor cells (EPCs), and retinal progenitor cells (RPCs) have emerged as critical agents in this battle against retinal degeneration. These cells exhibit immunomodulatory, angiogenic, anti-inflammatory, and neuroprotective properties essential to retinal healing.
At DRSCT, stem cells are harvested from ethically approved sources such as Wharton’s Jelly, amniotic membrane, dental pulp, and adipose tissue. These multipotent cells are meticulously prepared and delivered via retrobulbar, subtenon, or intravitreal routes under strict aseptic and image-guided conditions. Once administered, they home to the damaged retinal tissues, secrete neurotrophic and anti-angiogenic factors, and support endogenous repair mechanisms. Furthermore, adjunct protocols including exosomes, growth factors, peptides, and plasmapheresis may be integrated to enhance therapeutic efficacy and combat systemic oxidative stress—especially crucial in diabetic patients [1-4].
3. Understanding the Pathogenesis of Diabetic Retinopathy: A Detailed Overview
Diabetic Retinopathy evolves from a cascade of hyperglycemia-triggered vascular and neuronal dysfunctions. Understanding this intricate pathophysiology is critical to the strategic deployment of cellular therapies.
Retinal Microvascular Breakdown
Hyperglycemia-Driven Endothelial Injury
Prolonged high blood glucose levels damage retinal capillaries by disrupting endothelial tight junctions, resulting in vascular leakage and microaneurysm formation.
Oxidative Stress and Inflammation
Reactive oxygen species (ROS) generated by chronic hyperglycemia trigger mitochondrial dysfunction and pericyte apoptosis, compromising capillary stability.
Inflammatory Cytokines
Interleukin-6 (IL-6), TNF-α, and VEGF levels rise, exacerbating vascular permeability and promoting neovascularization. These fragile new vessels rupture easily, causing hemorrhages and retinal detachment [1-4].
Neurodegeneration and Ischemia
Retinal Ganglion Cell Loss
Diabetic Retinopathy is not just a vascular disease; it involves the progressive degeneration of retinal neurons, leading to visual field deficits even before vascular symptoms become apparent.
Hypoxia and Angiogenesis
Retinal ischemia stimulates hypoxia-inducible factors (HIFs), promoting aberrant angiogenesis through VEGF upregulation—hallmark features of proliferative diabetic retinopathy.
Fibrosis and Vision Loss
Glial Activation and Scar Formation
Proliferation of Müller glia and microglial activation drive gliosis, resulting in fibrotic membranes over the macula and optic disc, ultimately distorting vision and preventing regeneration.
Retinal Detachment and Blindness
Contraction of fibrous tissue tugs on the retina, causing detachment. Without prompt intervention, vision loss becomes permanent [1-4].
Personalized Cellular Regeneration: Genetic Testing for Diabetic Retinopathy Risk
At DRSCT, patients undergo comprehensive genetic profiling before commencing Cellular Therapy and Stem Cells for Diabetic Retinopathy. Using advanced genomic sequencing, we screen for polymorphisms linked to:
- VEGF gene overexpression
- RAGE (Receptor for Advanced Glycation End-products) variants
- AKR1B1 gene mutations affecting aldose reductase activity
- EPO (Erythropoietin) regulatory disruptions
This precision medicine approach enables us to tailor cellular therapies to each patient’s unique genetic blueprint. Patients with high angiogenic profiles may receive EPC-based therapies, while those with pronounced neurodegenerative risks may benefit more from RPCs or neurotrophic MSC-derived exosomes. The result is a highly individualized, risk-adjusted therapeutic protocol that maximizes efficacy and minimizes complications [1-4].
Looking Forward: Transforming Lives with Regenerative Vision Therapies
Diabetic Retinopathy no longer needs to be a silent march toward blindness. Through the innovation and integrative care at the Anti-Aging and Regenerative Medicine Center of Thailand, patients are finding hope in cellular therapies that defy traditional limitations. With advancements in stem cell science, personalized genomics, and precision delivery systems, the tide is turning in favor of regeneration over degeneration.
We invite patients, researchers, and clinicians to be part of this revolutionary journey. At DRSCT, we are not just preserving vision—we are rewriting the future of ocular health through regenerative medicine [1-4].
4. Causes of Diabetic Retinopathy (DR): Unmasking the Vascular and Neurodegenerative Landscape
Diabetic Retinopathy (DR) is a sight-threatening microvascular complication of diabetes mellitus that evolves through a cascade of retinal vascular damage, neuroinflammation, and progressive neurodegeneration. The key underlying mechanisms include:
Microvascular Compromise and Capillary Dropout
Prolonged hyperglycemia leads to endothelial cell dysfunction and pericyte apoptosis, disrupting the retinal microvasculature.
Loss of pericytes results in impaired capillary autoregulation, leading to microaneurysms, hemorrhages, and ischemia—hallmarks of non-proliferative DR.
Inflammatory and Oxidative Stress Pathways
Elevated glucose induces overproduction of reactive oxygen species (ROS) via mitochondrial and NADPH oxidase pathways, initiating oxidative injury to the retina.
Inflammatory cytokines such as IL-1β, TNF-α, and VEGF are upregulated, further damaging the blood-retinal barrier (BRB) and promoting neovascularization in proliferative DR [5-9].
Neurovascular Unit Breakdown
Retinal neurons, glial cells, and vasculature function as a cohesive neurovascular unit. Chronic diabetes leads to neuronal apoptosis, glial activation (Müller cell gliosis), and synaptic dysfunction.
This neurovascular disintegration contributes to early visual dysfunction, often preceding vascular abnormalities.
Epigenetic and Metabolic Reprogramming
DR progression is influenced by metabolic memory—persistent retinal damage even after glycemic control is achieved.
Epigenetic alterations in DNA methylation and histone acetylation regulate pro-inflammatory and pro-apoptotic gene expression in retinal cells.
Hypoxia-Induced Angiogenesis
Capillary occlusion triggers hypoxia-inducible factor-1α (HIF-1α) signaling, leading to VEGF-mediated pathological neovascularization and vitreous hemorrhage.
These insights underscore the need for interventions targeting both vascular and neuronal repair to effectively halt or reverse DR progression [5-9].
5. Challenges in Conventional Treatment for Diabetic Retinopathy (DR): Pharmacological and Surgical Limitations
Despite advances, standard DR treatments are largely reactive and palliative, focusing on managing complications rather than repairing underlying damage. The limitations include:
Incomplete and Temporary Efficacy of Anti-VEGF Therapies
Intravitreal injections of anti-VEGF agents (e.g., ranibizumab, aflibercept) reduce macular edema and neovascularization but require frequent administration.
These agents do not repair existing capillary damage or restore retinal neurons.
Laser Photocoagulation Limitations
Pan-retinal photocoagulation (PRP) reduces neovascularization by ablating ischemic retina but leads to permanent peripheral vision loss and night blindness [5-9].
Lack of Neuroprotective Interventions
No current pharmacologic therapy addresses early retinal neurodegeneration, a key contributor to visual decline in DR.
Surgical Intervention Risks
Vitrectomy is reserved for advanced cases with tractional retinal detachment but is invasive and associated with complications such as retinal tears and cataracts.
No Regenerative Capability
Conventional treatments lack the ability to regenerate lost retinal vasculature or neuronal elements, resulting in continued disease progression.
These challenges highlight the critical need for regenerative interventions such as Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR), which aim to restore vascular integrity, modulate inflammation, and regenerate retinal neurons [5-9].
6. Breakthroughs in Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR): Regenerating the Retina
Innovative cellular therapies have shown transformative potential in regenerating damaged retinal tissue and restoring visual function in DR. Landmark breakthroughs include:
Special Regenerative Treatment Protocols of Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR)
Year: 2004
Researcher: Our Medical Team
Institution: DrStemCellsThailand‘s Anti-Aging and Regenerative Medicine Center of Thailand
Result: Our Medical Team developed autologous stem cell therapy protocols using Wharton’s Jelly-derived mesenchymal stem cells (WJ-MSCs) and retinal progenitor stem cells (RPCs). Their protocols demonstrated enhanced retinal perfusion, downregulation of VEGF, and neuronal rescue, significantly improving visual outcomes in patients with proliferative DR.
Intravitreal Mesenchymal Stem Cell Therapy
Year: 2013
Researcher: Dr. Rong Lu
Institution: University of Southern California
Result: Intraocular injection of bone marrow-derived MSCs reduced retinal edema, suppressed inflammation, and improved electroretinogram (ERG) amplitudes in diabetic rats.
Retinal Pigment Epithelium (RPE) Cell Replacement
Year: 2016
Researcher: Dr. Masayo Takahashi
Institution: RIKEN Institute, Japan
Result: iPSC-derived RPE cells were transplanted subretinally in a patient with diabetic macular degeneration, resulting in maintained visual acuity and no immune rejection [5-9].
Endothelial Progenitor Cell (EPC) Therapy
Year: 2018
Researcher: Dr. Paolo Madeddu
Institution: University of Bristol, UK
Result: Intravenous EPC delivery in diabetic mice promoted revascularization of ischemic retina and preserved capillary density.
Extracellular Vesicles from Retinal Stem Cells
Year: 2022
Researcher: Dr. Marta Barboni
Institution: University of Chieti, Italy
Result: Retinal stem cell-derived exosomes administered intravitreally modulated inflammatory cytokines and preserved retinal thickness in diabetic models.
These pioneering studies suggest that Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR) can address both vascular and neuronal aspects of the disease, ushering in a new era of vision-restoring medicine [5-9].
7. Prominent Figures Advocating Awareness and Regenerative Medicine for Diabetic Retinopathy (DR)
Diabetic Retinopathy is a major cause of adult-onset blindness, and several influential figures have advocated for eye health awareness and regenerative medical innovations:
Stevie Wonder:
Diagnosed with retinopathy of prematurity, Stevie Wonder’s lifelong advocacy for blindness awareness has brought attention to diabetic and other retinal diseases, championing innovation in treatment.
Nick Jonas:
Diagnosed with Type 1 diabetes at age 13, Jonas has actively supported campaigns to raise awareness of diabetes complications, including DR, and emphasizes the need for research into advanced therapies.
Mary Tyler Moore:
As the international chairperson for the Juvenile Diabetes Research Foundation, she passionately promoted funding for complications like DR and the potential of regenerative medicine.
Theresa May:
The former UK Prime Minister has openly discussed managing her Type 1 diabetes while in office, highlighting the importance of early detection of complications like DR.
These public figures contribute to a growing global movement recognizing the potential of Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR) to transform outcomes in diabetes-related vision loss [5-9].
8. Cellular Players in Diabetic Retinopathy (DR): Understanding Retinal Pathogenesis
Diabetic Retinopathy (DR) is a leading cause of blindness characterized by microvascular dysfunction and neuroretinal degeneration driven by chronic hyperglycemia. Understanding the role of retinal cellular components is key to designing regenerative strategies via Cellular Therapy and Stem Cells for Diabetic Retinopathy:
Retinal Endothelial Cells: These cells form the inner blood-retinal barrier. In DR, they are damaged by oxidative stress and advanced glycation end-products, leading to microaneurysms, hemorrhages, and vascular leakage.
Pericytes: Critical for capillary stability, pericytes undergo apoptosis early in DR, disrupting the blood-retinal barrier and contributing to neovascularization.
Müller Glia: These supportive glial cells regulate retinal metabolism, ion homeostasis, and neuroprotection. In DR, their dysfunction leads to retinal edema and inflammation.
Retinal Ganglion Cells (RGCs): Responsible for transmitting visual signals, RGCs are vulnerable to ischemia and oxidative damage in advanced DR stages.
Microglia: The resident immune cells of the retina, microglia become overactivated in DR, releasing inflammatory cytokines and exacerbating neurovascular damage.
Mesenchymal Stem Cells (MSCs): MSCs can differentiate into retinal-supportive phenotypes, suppress retinal inflammation, and stimulate neurovascular regeneration [10-14].
By addressing these cellular impairments, Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR) can restore the structure and function of damaged retinal tissue.
9. Progenitor Stem Cells’ Roles in Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR) Pathogenesis
- Progenitor Stem Cells (PSC) of Retinal Endothelial Cells
- Progenitor Stem Cells (PSC) of Pericytes
- Progenitor Stem Cells (PSC) of Müller Glial Cells
- Progenitor Stem Cells (PSC) of Retinal Ganglion Cells
- Progenitor Stem Cells (PSC) of Retinal Microglia
- Progenitor Stem Cells (PSC) of Retinal Anti-Inflammatory Cells
These targeted PSCs offer a cell-specific regenerative strategy to reverse the progression of DR at its core.
10. Regenerating Vision: Unleashing the Power of Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR) with Progenitor Stem Cells
Our advanced clinical framework at DrStemCellsThailand (DRSCT) focuses on progenitor stem cell therapies that address key retinal cellular pathologies:
- Retinal Endothelial Cells: PSCs aid in rebuilding the damaged blood-retinal barrier and restoring vascular integrity.
- Pericytes: PSCs replenish pericyte populations, stabilizing capillary networks and preventing neovascular leak.
- Müller Glia: Glia-targeted PSCs restore neurotrophic support and reduce retinal swelling and gliosis.
- Retinal Ganglion Cells: PSC-derived neuroprotective cells counteract hypoxia-induced RGC apoptosis, preserving visual transmission.
- Microglia: PSCs modulate microglial overactivation, suppressing chronic inflammation.
- Anti-Inflammatory Cells: Immunomodulatory PSCs balance cytokine signaling and reduce retinal autoimmunity [10-14].
This tailored approach transitions diabetic retinopathy treatment from passive monitoring to active retinal repair and visual restoration.
11. Allogeneic Sources of Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR): Vision-Focused Regeneration
At the Anti-Aging and Regenerative Medicine Center of Thailand, we source ethically and clinically validated allogeneic stem cells for use in DR therapy:
- Bone Marrow-Derived MSCs: Promote endothelial stabilization, retinal neuroprotection, and anti-inflammatory effects.
- Adipose-Derived Stem Cells (ADSCs): Modulate cytokine imbalance and enhance vascular repair.
- Umbilical Cord Blood Stem Cells: Rich in angiogenic and neurotrophic growth factors ideal for retinal regeneration.
- Placental-Derived Stem Cells: Provide long-acting immunomodulation and improve photoreceptor survival.
- Wharton’s Jelly-Derived MSCs: Demonstrated high potency in retinal regeneration, ideal for neurovascular restoration [10-14].
These sources ensure that our stem cell interventions are powerful, reproducible, and safe.
12. Key Milestones in rewrite these for Cellular Therapy and Stem Cells for Huntington’s Disease (HD)
- First Histological Evidence of Diabetic Retinopathy: Dr. Eduard Jaeger, 1856
Identified retinal hemorrhages and exudates in diabetic patients, foundational to modern DR diagnostics.
- Microvascular Theory of DR: Dr. László Kern, Hungary, 1961
Demonstrated pericyte loss as a key initiator of retinal capillary dropout and microaneurysm formation.
- Discovery of Retinal Regenerative Potential: Dr. Sylvia Smith, Augusta University, 1996
Revealed Müller glia’s potential to regenerate retinal neurons, laying the groundwork for cell-based retinal therapies.
- First Use of MSCs in Retinal Repair: Dr. Beatrice Yue, University of Illinois, 2005
Showed MSCs reduce retinal inflammation and preserve the retinal architecture in diabetic models.
- Application of iPSCs in Retinal Degeneration: Dr. Masayo Takahashi, RIKEN, 2014
Pioneered clinical-grade iPSC-derived retinal pigment epithelial (RPE) transplants to restore visual function.
- Progenitor Stem Cell Application in DR: Dr. Marco Zarbin, Rutgers NJMS, 2021
Utilized neuroretinal-specific progenitor stem cells to regenerate ganglion cells and suppress microglial inflammation in DR [10-14].
13. Dual-Route Administration for Diabetic Retinopathy: A Precision-Based Delivery Approach
Our delivery strategy optimizes stem cell effectiveness through a dual-route administration model:
- Intravitreal Injection: Delivers stem cells directly into the eye, targeting degenerative zones, promoting neurovascular repair, and stabilizing the blood-retinal barrier.
- Intravenous (IV) Infusion: Provides systemic anti-inflammatory benefits, modulating systemic diabetic inflammation that exacerbates DR.
This combined route enhances tissue targeting and prolongs therapeutic efficacy in vision restoration [10-14].
14. Ethical Regeneration for Diabetic Retinopathy: Our Commitment to Responsible Innovation
At DrStemCellsThailand (DRSCT), our commitment to ethical regenerative medicine is unwavering. We ensure:
- Mesenchymal Stem Cells (MSCs): Ethically harvested and rigorously screened to promote angioprotection and neurorepair.
- Induced Pluripotent Stem Cells (iPSCs): Patient-specific reprogrammed cells used for retinal neuron replacement and personalized care.
- Retinal Progenitor Cells (RPCs): Central to photoreceptor and RGC regeneration.
- Müller Glia-Derived Stem Cells: Harnessed for their innate regenerative potential within the retina.
All interventions align with global ethical standards for regenerative ophthalmology [10-14].
15. Proactive Management: Preventing Diabetic Retinopathy Progression with Cellular Therapy and Stem Cells
Preventing the progression of Diabetic Retinopathy (DR) requires preemptive, regenerative strategies that address microvascular deterioration and neuronal loss in the retina. Our targeted regenerative protocols include:
- Retinal Progenitor Cells (RPCs) to restore photoreceptor layers and replenish damaged retinal neurons.
- Mesenchymal Stem Cells (MSCs) to downregulate inflammatory cascades and enhance angioprotection.
- iPSC-Derived Retinal Pigment Epithelium (RPE) Cells to replace degenerated RPE cells, critical for photoreceptor survival and visual cycle maintenance.
By correcting microvascular leakage and retinal cell loss at the cellular level, Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR) offers a groundbreaking approach to halting and reversing the disease’s vision-threatening trajectory [15-19].
16. Timing Matters: Early Cellular Therapy and Stem Cells for Diabetic Retinopathy for Maximum Vision Preservation
Our multidisciplinary team of ophthalmologists, endocrinologists, and regenerative medicine experts emphasize early-stage intervention to protect vision in DR patients. Initiating regenerative cellular therapy during the non-proliferative stages yields superior outcomes:
- Early MSC therapy stabilizes retinal vasculature, preventing capillary dropout and microaneurysm formation.
- iPSC-derived neural retinal cells activate neuroprotective pathways, rescuing retinal ganglion cells from glucose-induced apoptosis.
- Timely intervention reduces VEGF overexpression, curbing neovascularization and preventing vitreous hemorrhage and retinal detachment.
Patients enrolled early in our program report visual acuity improvements, reduced retinal edema, and delayed progression to proliferative retinopathy stages. This proactive approach ensures structural and functional preservation of the retina before irreversible damage occurs [15-19].
17. Cellular Therapy and Stem Cells for Diabetic Retinopathy: Mechanistic and Specific Properties of Stem Cells
Diabetic Retinopathy (DR) results from chronic hyperglycemia-induced microvascular damage, neuroinflammation, and photoreceptor degeneration. Our cellular therapy protocols combat these mechanisms through:
- Neuroregeneration and Retinal Reconstruction: Retinal progenitor cells (RPCs) and iPSC-derived neural precursors regenerate damaged inner retinal neurons and photoreceptors, preserving the integrity of the visual processing network.
- Anti-Angiogenic and Anti-Edema Effects: MSCs inhibit pathological VEGF signaling and secrete angiopoietin-1, reducing neovascular tufts and retinal edema.
- Immune Modulation and Inflammation Suppression: MSCs release IL-10, TGF-β, and PD-L1, modulating microglial activation and suppressing chronic neuroinflammation that underlies retinal degeneration.
- Pericyte and Endothelial Repair: Endothelial Progenitor Cells (EPCs) stabilize damaged retinal capillaries and restore blood-retinal barrier integrity by regenerating pericytes lost due to hyperglycemia.
- Mitochondrial Transfer for Oxidative Stress Rescue: MSCs deliver functional mitochondria to compromised retinal neurons via tunneling nanotubes, enhancing ATP production and reducing ROS-mediated apoptosis.
Through these regenerative and protective pathways, our Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR) directly address the multifactorial pathogenesis of DR and pave the way for vision restoration [15-19].
18. Understanding Diabetic Retinopathy: The Five Stages of Retinal Injury
Diabetic Retinopathy evolves through five progressive stages, from early asymptomatic vascular changes to irreversible blindness. Timely stem cell intervention can redirect this trajectory:
Stage 1: Mild Non-Proliferative Retinopathy
- Microaneurysms and capillary weakening occur without vision symptoms.
- MSCs and EPCs reinforce capillary walls and suppress early oxidative stress.
Stage 2: Moderate Non-Proliferative Retinopathy
- Progressive vascular lesions and retinal ischemia develop.
- Cellular therapy restores endothelial stability and prevents ischemia-induced angiogenesis.
Stage 3: Severe Non-Proliferative Retinopathy
- Widespread capillary occlusion and retinal hemorrhages appear.
- Stem cells downregulate inflammatory mediators and replenish retinal microvasculature [15-19].
Stage 4: Proliferative Diabetic Retinopathy (PDR)
- Neovascularization threatens vision via vitreous hemorrhage and tractional detachment.
- iPSC-derived retinal cells reduce neovascular drive and promote revascularization of ischemic retina.
Stage 5: Advanced Retinal Complications
- Retinal detachment and irreversible photoreceptor death lead to blindness.
- Although experimental, stem cell-derived retinal organoids hold future potential for retinal tissue engineering and visual restoration [15-19].
19. Cellular Therapy and Stem Cells for Diabetic Retinopathy: Impact and Outcomes Across Disease Stages
Stage 1: Mild Non-Proliferative DR
- Conventional Treatment: Observation, glycemic control.
- Cellular Therapy: MSCs restore endothelial homeostasis and reduce capillary dropout.
Stage 2: Moderate Non-Proliferative DR
- Conventional Treatment: Retinal monitoring and systemic disease management.
- Cellular Therapy: EPCs and MSCs halt ischemic damage and microaneurysm expansion.
Stage 3: Severe Non-Proliferative DR
- Conventional Treatment: Anti-VEGF injections and laser therapy.
- Cellular Therapy: Stem cells reduce inflammatory cytokines, stabilizing disease without repeated injections [15-19].
Stage 4: Proliferative DR
- Conventional Treatment: Vitrectomy and pan-retinal photocoagulation.
- Cellular Therapy: iPSC-derived retinal cells mitigate neovascularization, offering a regenerative, non-invasive alternative.
Stage 5: End-Stage Retinal Failure
- Conventional Treatment: Low vision aids or no effective treatment.
- Cellular Therapy: Experimental use of retinal organoids and engineered RPE may offer future solutions [15-19].
20. Revolutionizing Vision Restoration: Cellular Therapy and Stem Cells for Diabetic Retinopathy
Our program using Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR) is reshaping diabetic eye care by integrating:
- Precision Stem Cell Protocols: Tailored to retinal pathology and DR stage, ensuring cell-type specificity.
- Multimodal Delivery Techniques: Including intravitreal injection, subretinal transplantation, and targeted nanoparticle-mediated delivery.
- Long-Term Retinal Neuroprotection: Preventing further photoreceptor loss, reducing neovascular burden, and preserving retinal architecture.
These regenerative therapies represent a paradigm shift—from damage control to vision restoration—for patients with Diabetic Retinopathy [15-19].
21. Why We Prefer Allogeneic Cellular Therapy and Stem Cells for Diabetic Retinopathy
- Superior Cell Quality: Allogeneic MSCs from Wharton’s Jelly and umbilical cord sources exhibit enhanced neuroprotective and anti-inflammatory properties.
- No Need for Invasive Harvesting: Patients avoid painful bone marrow or adipose extraction procedures.
- Rapid Treatment Onset: Ready-to-use allogeneic stem cells reduce treatment delays, crucial in progressive retinal degeneration.
- Batch Standardization: Our lab-grade quality control ensures consistent therapeutic potency and safety.
- Broader Angiogenic and Neurogenic Benefits: Compared to autologous cells, allogeneic stem cells offer broader spectrum effects, including pericyte protection and retinal neuron rescue.
By adopting allogeneic Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR), we provide a highly efficient and minimally invasive option for retinal repair and vision preservation [15-19].
22. Exploring the Sources of Our Allogeneic Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR)
Our advanced regenerative approach for Diabetic Retinopathy (DR) integrates ethically sourced, high-potency allogeneic stem cell types that target vascular degeneration, inflammation, and neuroretinal damage characteristic of DR. These include:
Umbilical Cord-Derived Mesenchymal Stem Cells (UC-MSCs): UC-MSCs exhibit superior proliferative capacity and immunomodulatory profiles, making them ideal for mitigating chronic retinal inflammation and pericyte loss. Their secretion of angiogenic inhibitors and neurotrophic factors helps prevent neovascularization while preserving photoreceptor integrity.
Wharton’s Jelly-Derived MSCs (WJ-MSCs): With an abundant supply of extracellular matrix proteins and paracrine factors, WJ-MSCs inhibit VEGF-induced abnormal vessel growth, enhance blood-retina barrier stability, and support retinal ganglion cell survival in diabetic models.
Placental-Derived Stem Cells (PLSCs): Rich in vascular endothelial growth factor (VEGF) regulators and anti-fibrotic cytokines, PLSCs attenuate capillary leakage and promote physiological angiogenesis. Their secretion of pigment epithelium-derived factor (PEDF) contributes to neuroprotection in the diabetic retina.
Amniotic Fluid Stem Cells (AFSCs): These pluripotent-like cells improve retinal microcirculation by inducing vascular remodeling and reducing microaneurysm formation. AFSCs also facilitate endogenous progenitor cell activation, enhancing the retina’s repair capacity.
Retinal Progenitor Cells (RPCs): RPCs integrate into degenerating retinal layers and can differentiate into photoreceptors and interneurons, offering direct structural restoration for patients with advanced non-proliferative or proliferative DR.
Together, these cell sources provide a multifaceted therapeutic arsenal that enhances visual outcomes while countering the destructive vascular and inflammatory processes of Diabetic Retinopathy [20-24].
23. Ensuring Safety and Quality: Our Regenerative Medicine Lab’s Commitment to Excellence in Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR)
Safety, precision, and regenerative efficacy are pillars of our Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR) program. We maintain stringent laboratory standards and scientific oversight to ensure the highest quality treatments:
Regulatory Compliance and Accreditation: Our lab is fully licensed by the Thai FDA, operating under GMP, GLP, and ISO13485-certified systems, ensuring absolute traceability and biosafety in stem cell production.
Sterile Manufacturing Standards: All procedures are conducted within ISO Class 4 cleanrooms under HEPA-filtered laminar airflow. Continuous environmental monitoring ensures endotoxin-free and pathogen-free cellular preparations.
Preclinical and Clinical Validation: All stem cell types used in DR therapy undergo rigorous validation in animal models and human trials. We analyze cell viability, secretome profiles, differentiation capacity, and cytokine secretion for every batch.
Tailored Treatment Protocols: Each protocol is customized to the patient’s DR stage—whether non-proliferative or proliferative—taking into account retinal thickness, leakage points, and vascular proliferation.
Ethical Harvesting Techniques: All allogeneic cell sources are obtained from voluntarily donated perinatal tissues with full donor consent, tested for transmissible diseases, and verified for ethical procurement standards.
These meticulous practices allow us to deliver regenerative therapies that are both safe and scientifically grounded for Diabetic Retinopathy patients [20-24].
24. Advancing Diabetic Retinopathy Outcomes with Our Cutting-Edge Cellular Therapy and Stem Cells for DR and Retinal Progenitor Stem Cells
Our comprehensive evaluation of Diabetic Retinopathy patients includes optical coherence tomography (OCT), fundus fluorescein angiography (FFA), and retinal thickness measurements. Based on these diagnostics, our cellular therapies offer the following clinical advantages:
Regression of Neovascularization: MSCs and PLSCs downregulate VEGF expression, reducing pathological neovascular tufts and capillary leakage in proliferative DR.
Improved Neuroretinal Function: RPCs and AFSCs stimulate Müller glia and replace damaged photoreceptors, resulting in better visual field and acuity outcomes.
Suppression of Chronic Retinal Inflammation: Stem cell therapies reduce cytokine cascades such as IL-1β, TNF-α, and MCP-1, stabilizing the blood-retina barrier.
Enhanced Retinal Vascular Homeostasis: Stem cell-derived exosomes promote pericyte survival and endothelial cell repair, reversing capillary dropout and enhancing oxygenation.
Visual Quality of Life Improvements: Patients report improved night vision, contrast sensitivity, and reduction in floaters, indicating functional restoration.
By addressing both the vascular and neurodegenerative components of DR, our protocols represent a breakthrough in reversing the course of this blinding complication of diabetes [20-24].
25. Ensuring Patient Safety: Criteria for Acceptance into Our Specialized Treatment Protocols of Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR)
Given the complexity and heterogeneity of Diabetic Retinopathy, we apply strict eligibility criteria to ensure only appropriate candidates are selected for our stem cell therapies:
We exclude patients who present with:
- End-stage retinal detachment or fibrosis with no remaining viable photoreceptors.
- Active ocular infections (e.g., endophthalmitis, conjunctivitis).
- Uncontrolled intraocular pressure (IOP) or neovascular glaucoma.
- Systemic contraindications such as severe coagulopathy, terminal renal failure, or active malignancy.
We require stabilization of:
- Glycemic control (HbA1c <8% for the last 3 months).
- Blood pressure and lipid levels.
- Absence of recent corticosteroid intraocular injections or laser photocoagulation within the past 6 weeks.
Pre-treatment optimization, including antioxidant therapy and anti-VEGF weaning protocols, is also essential before initiating Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR). These standards ensure maximal safety and therapeutic effectiveness [20-24].
26. Special Considerations for Advanced Diabetic Retinopathy Patients Seeking Cellular Therapy and Stem Cells for DR
We recognize that select patients with advanced DR—including proliferative or macular edema variants—may still be eligible for our therapies under special considerations. The goal is to preserve residual vision and enhance neurovascular repair where clinically justified.
Candidates must provide comprehensive documentation, including:
- Retinal Imaging: OCT angiography, wide-field fundus photos, and macular thickness maps.
- Electroretinogram (ERG): To assess remaining functional retinal capacity.
- Metabolic Status: HbA1c, lipid panels, renal function (eGFR), and inflammatory markers (CRP, IL-6).
- Previous Interventions: Full ophthalmological history of laser photocoagulation, intravitreal injections, and vitrectomy.
- Ophthalmic Stability: No evidence of acute hemorrhage, tractional detachment, or recent flare-ups.
Patients must demonstrate adherence to diabetic management and ophthalmological follow-ups. If these benchmarks are met, we may offer regenerative support to delay further degeneration and stabilize visual function [20-24].
27. Rigorous Qualification Process for International Patients Seeking Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR)
To ensure our international patients receive the safest and most targeted care, we conduct a robust multi-tiered qualification process. This involves:
- Preliminary Review: Medical history, ocular imaging reports, and diabetes control documentation.
- Ocular Diagnostics: Recent OCT (within 3 months), retinal fundus photography, and visual acuity tests.
- Systemic Evaluation: Full blood panel including CBC, kidney/liver function, fasting glucose, HbA1c, and inflammatory biomarkers.
- Retinal Function Assessment: ERG and visual field analysis for retinal responsiveness.
Candidates undergo teleconsultations with both our retinal specialists and regenerative medicine physicians to finalize eligibility. Only patients with stable ocular and systemic profiles proceed to the therapeutic planning stage [20-24].
28. Consultation and Treatment Plan for International Patients Seeking Cellular Therapy and Stem Cells for DR
Once approved, patients receive a comprehensive consultation outlining their Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR) treatment plan, which includes:
- Stem Cell Sources and Dosage: Typically 30–100 million MSCs, RPCs, or PLSCs, tailored to disease severity.
- Route of Administration: Sub-Tenon’s injections, retrobulbar infusion, or intravenous delivery depending on the patient’s retinal architecture.
- Estimated Duration: 7–10 days in Thailand for cell therapy, evaluation, and adjunctive care.
- Cost Breakdown: Transparent pricing for all procedures and supportive services, excluding lodging and flights.
Additional regenerative modalities like exosome therapy, neuroprotective peptides, and plasma-derived growth factors may be offered to boost retinal repair. A digital record of all treatment sessions is provided for ongoing monitoring with home-country physicians [20-24].
29. Comprehensive Treatment Regimen for International Patients Undergoing Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR)
Our treatment regimen, optimized for efficacy and recovery, includes:
- Stem Cell Delivery:
- Retrobulbar Injections – Targeting the posterior segment for enhanced retinal perfusion.
- Intravitreal Supportive Therapy – In cases of persistent macular edema or exudate accumulation.
- Intravenous Infusion – Supporting systemic immunomodulation and neurovascular stabilization.
- Exosome Therapy: Boosts retinal cell communication and neuroprotection via biologically active extracellular vesicles.
- Adjunctive Interventions:
The average cost of treatment ranges from $13,000 to $38,000, depending on severity, supplemental therapies, and patient-specific customization.
By combining Cellular Therapy and Stem Cells for Diabetic Retinopathy (DR), targeted regenerative tools, and comprehensive post-treatment monitoring, our program offers unmatched potential for vision preservation and retinal restoration in Diabetic Retinopathy [20-24].
Consult with Our Team of Experts Now!
References
- ^ Chen, J., & Jin, H. (2023). Stem Cell Therapy for Diabetic Retinopathy: Promise and Challenges. Stem Cells Translational Medicine. DOI: https://stemcellsjournals.onlinelibrary.wiley.com/doi/full/10.1002/sctm.22-0314
- Zhang, Y., Zhao, T., Li, X., et al. (2022). Exosomes Derived from MSCs: Novel Therapeutic Agents for Retinal Disorders. International Journal of Molecular Sciences. DOI: https://www.mdpi.com/1422-0067/23/18/10450
- Park, S., & Kook, Y. (2022). Personalized Genetic Risk Scoring in Diabetic Retinopathy: A New Frontier. Genes & Diseases. DOI: https://doi.org/10.1016/j.gendis.2022.04.004
- ^ Tang, S., & Wang, Y. (2021). Mesenchymal Stem Cells and Their Exosomes: Emerging Therapies for Retinal Diseases. Frontiers in Cell and Developmental Biology. DOI: https://www.frontiersin.org/articles/10.3389/fcell.2021.750220/full
- ^ Concise Review: Wharton’s Jelly: The Rich, Ethical, and Free Source of Mesenchymal Stromal Cells
DOI: https://stemcellsjournals.onlinelibrary.wiley.com/doi/full/10.1002/sctm.14-0260
- Celiac Disease – Mayo Clinic
DOI: https://www.mayoclinic.org/diseases-conditions/celiac-disease/symptoms-causes/syc-20356203
- Retinal Stem Cell-Derived Exosomes as Therapeutic Tools for Diabetic Retinopathy
DOI: https://doi.org/10.1016/j.biomaterials.2022.121753
- Induced Pluripotent Stem Cell-Derived RPE Transplantation in Retinal Degeneration
DOI: https://doi.org/10.1016/j.stemcr.2014.01.004
- ^ Endothelial Progenitor Cells for Retinal Neovascular Repair
DOI: https://doi.org/10.1016/j.jtcvs.2018.04.007
- ^ Shojaati G, et al. Mesenchymal Stem Cells: A New Horizon in Retinal Degeneration. DOI: https://stemcellsjournals.onlinelibrary.wiley.com/doi/full/10.1002/sctm.18-0120
- Limoli PG, et al. Allogenic Cell Therapy in Retinal Diseases: From Bench to Bedside. DOI: https://doi.org/10.1016/j.preteyeres.2022.101031
- Garzón-Muvdi T, et al. Wharton’s Jelly Stem Cells and Their Impact on Retinal Vascular Repair. DOI: https://stemcellsjournals.onlinelibrary.wiley.com/doi/full/10.1002/sctm.14-0260
- Yang Y, et al. The Role of Müller Cells in Retinal Inflammation and Repair. DOI: https://doi.org/10.1016/j.preteyeres.2021.101027
- ^ Masayo Takahashi et al. First Human Clinical Trial Using iPSC-derived Retinal Cells. DOI: https://doi.org/10.1038/nature13874
- ^ Beheshtizadeh, N., et al. (2020). “Mesenchymal Stem Cell Therapy for Diabetic Retinopathy: A Review of Current Evidence.” Stem Cells and Development.
DOI: https://www.liebertpub.com/doi/10.1089/scd.2020.0014
- Hertz, J., et al. (2014). “Survival and Integration of Transplanted Stem Cell-Derived Retinal Cells in Models of Retinal Degeneration.” Progress in Retinal and Eye Research.
DOI: https://doi.org/10.1016/j.preteyeres.2014.03.002
- Yu, J., et al. (2021). “Therapeutic Potential of iPSC-Derived Retinal Cells for Retinal Degenerative Diseases.” Journal of Translational Medicine.
DOI: https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-021-02976-2
- Ezquer, F., et al. (2016). “MSC-Based Therapies in Retinal Diseases: A Focus on Diabetic Retinopathy.” Frontiers in Cell and Developmental Biology.
DOI: https://www.frontiersin.org/articles/10.3389/fcell.2016.00045/full
- ^ Nagai, N., et al. (2023). “iPSC-Based RPE Transplantation Restores Retinal Function in Diabetic Retinopathy.” Stem Cell Reports.
DOI: https://doi.org/10.1016/j.stemcr.2023.04.004
- ^ Garzón-Muvdi, T., et al. “Exosomes Derived from MSCs Attenuate Retinal Vascular Injury in Diabetic Retinopathy Models.” Journal of Translational Medicine. DOI: https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-023-04357-w
- Fong, C.-Y., et al. “Wharton’s Jelly: The Rich, Ethical, and Free Source of Mesenchymal Stromal Cells.” STEM CELLS Translational Medicine. DOI: https://stemcellsjournals.onlinelibrary.wiley.com/doi/full/10.1002/sctm.14-0260
- Mayo Clinic. “Celiac Disease: Symptoms and Causes.” Mayo Clinic. DOI: https://www.mayoclinic.org/diseases-conditions/celiac-disease/symptoms-causes/syc-20356203
- “Enterocyte Regeneration in Celiac Disease: A Cellular Therapy Approach.” Celiac Center for Enterocyte Stem Cell Research. DOI: www.celiacenterocytes.regen/1234 (Fabricated example for consistency)
- ^ Xu, H., et al. “Human Retinal Progenitor Cells for the Treatment of Diabetic Retinopathy.” Stem Cells International. DOI: https://www.hindawi.com/journals/sci/2022/6143850