Cellular Therapy and Stem Cells for Chronic Pancreatitis represent a groundbreaking advancement in regenerative medicine, offering innovative therapeutic strategies for this debilitating pancreatic disorder. Chronic Pancreatitis is characterized by persistent inflammation, progressive fibrosis, and irreversible damage to pancreatic tissue, leading to exocrine and endocrine dysfunction. Conventional treatments, such as enzyme replacement therapy, analgesics, and surgical interventions, primarily focus on symptom management rather than reversing pancreatic damage. This introduction will explore the potential of Cellular Therapy and Stem Cells for Chronic Pancreatitis to regenerate pancreatic tissue, reduce inflammation, and enhance pancreatic function, presenting a transformative approach to treating this chronic disease. Recent scientific advancements and future directions in this evolving field will be highlighted.
Despite progress in gastroenterology, conventional treatments for Chronic Pancreatitis remain limited in their ability to restore pancreatic function and prevent disease progression. Standard approaches, including pharmacological interventions and dietary modifications, primarily target symptoms without addressing the underlying pathology—acinar cell injury, oxidative stress, inflammation, and fibrosis. Consequently, many Chronic Pancreatitis patients continue to experience relentless pancreatic deterioration, increasing the risk of diabetes, malabsorption, and pancreatic cancer. These limitations underscore the urgent need for regenerative therapies that go beyond symptomatic management to actively restore pancreatic integrity and function [1-4].
The convergence of Cellular Therapy and Stem Cells for Chronic Pancreatitis represents a paradigm shift in gastroenterology. Imagine a future where the devastating effects of Chronic Pancreatitis can be halted or even reversed through regenerative medicine. This pioneering field holds the promise of not only alleviating symptoms but fundamentally changing the disease trajectory by promoting pancreatic repair and functional restoration at a cellular level. Join us as we explore this revolutionary intersection of gastroenterology, regenerative science, and cellular therapy, where innovation is redefining what is possible in the treatment of Chronic Pancreatitis.
2. Genetic Insights: Personalized DNA Testing for Chronic Pancreatitis Risk Assessment before Cellular Therapy and Stem Cells for Chronic Pancreatitis
Our team of pancreatic specialists and genetic researchers offers comprehensive DNA testing services for individuals with a family history of Chronic Pancreatitis. This service aims to identify specific genetic markers associated with hereditary predispositions to pancreatic damage and digestive enzyme disorders. By analyzing key genomic variations linked to serine protease inhibitor Kazal-type 1 (SPINK1), cystic fibrosis transmembrane conductance regulator (CFTR), chymotrypsin C (CTRC), and carboxypeptidase A1 (CPA1), we can better assess individual risk factors and provide personalized recommendations for preventive care before administering Cellular Therapy and Stem Cells for Chronic Pancreatitis. This proactive approach enables patients to gain valuable insights into their pancreatic health, allowing for early intervention through lifestyle modifications, targeted therapies, and pancreatic-protective strategies. With this information, our team can guide individuals toward optimal pancreatic health strategies that may significantly reduce the risk of Chronic Pancreatitis progression and its complications [1-4].
3. Understanding the Pathogenesis of Chronic Pancreatitis: A Detailed Overview
Chronic Pancreatitis is a complex pancreatic disorder resulting from persistent inflammation, leading to acinar cell injury, fibrosis, and endocrine dysfunction. The pathogenesis of Chronic Pancreatitis involves a multifaceted interplay of genetic, molecular, and inflammatory factors that contribute to pancreatic damage. Here is a detailed breakdown of the mechanisms underlying Chronic Pancreatitis:
Pancreatic Injury and Inflammation
Enzyme Activation and Acinar Cell Damage
- Inappropriate Trypsin Activation: Premature activation of trypsinogen within the pancreas leads to autodigestion and acinar cell injury.
- Oxidative Stress: Persistent inflammation results in excessive reactive oxygen species (ROS) production, causing lipid peroxidation and DNA damage.
- Mitochondrial Dysfunction: Impaired mitochondrial function reduces ATP production, promoting acinar cell apoptosis [1-4].
Inflammatory Cascade
- Macrophage and T-cell Activation: Chronic inflammation recruits immune cells, leading to sustained cytokine release.
- Fibrogenesis: Overactivation of pancreatic stellate cells (PSCs) drives excessive extracellular matrix deposition, leading to fibrosis.
- TNF-α and IL-6 Signaling: These pro-inflammatory cytokines perpetuate pancreatic injury and fibrosis.
Fibrosis and Endocrine Dysfunction Progression
Pancreatic Stellate Cell Activation
- Transforming Growth Factor-Beta (TGF-β) Signaling: TGF-β promotes fibroblast proliferation and collagen synthesis, driving pancreatic fibrosis.
- Loss of Exocrine Function: Progressive fibrosis leads to malabsorption and steatorrhea due to enzyme insufficiency [1-4].
Endocrine Dysfunction
- Islet Cell Damage: Chronic inflammation affects pancreatic islets, increasing the risk of diabetes mellitus.
- Beta-Cell Apoptosis: Oxidative stress and chronic inflammation contribute to beta-cell loss and insulin deficiency.
Pancreatic Cancer Risk
- Oncogenic Transformation: Chronic inflammation and fibrosis increase the risk of pancreatic ductal adenocarcinoma (PDAC).
- Genetic Mutations: Accumulation of genetic alterations in pancreatic cells promotes malignant transformation [1-4].
Overall, the pathogenesis of Chronic Pancreatitis is driven by a complex interplay of acinar cell injury, inflammatory responses, and fibrotic remodeling. Early identification and intervention targeting these pathways through Cellular Therapy and Stem Cells for Chronic Pancreatitis hold immense potential in reversing disease progression and restoring pancreatic function.
4. Revolutionizing Treatment: The Promise of Cellular Therapy and Stem Cells for Chronic Pancreatitis at DrStemCellsThailand (DRSCT)’s Anti-Aging and Regenerative Medicine Center of Thailand
Cellular Therapy and Stem Cells for Chronic Pancreatitis represent an innovative frontier in regenerative medicine, offering transformative therapeutic options for this debilitating pancreatic disorder. Chronic Pancreatitis is characterized by progressive inflammation, fibrosis, and loss of pancreatic function, often leading to exocrine and endocrine insufficiency. Conventional treatments, including enzyme replacement, pain management, and surgical interventions, offer symptomatic relief but fail to halt disease progression. This introduction will explore the potential of Cellular Therapy and Stem Cells for Chronic Pancreatitis to regenerate pancreatic tissues, modulate inflammation, and restore endocrine and exocrine functions, marking a revolutionary approach to treatment. Scientific advancements and future directions in this field will be highlighted.
Despite advances in gastroenterology, conventional treatments for Chronic Pancreatitis remain inadequate in reversing pancreatic damage. Current therapies primarily focus on symptom control rather than addressing the underlying pathology—fibrosis, acinar cell loss, and islet cell dysfunction. Consequently, patients continue to experience relentless disease progression, often culminating in pancreatic insufficiency and diabetes. These limitations underscore the urgent need for regenerative approaches that extend beyond symptomatic relief to actively restore pancreatic integrity and function [5-9].
The integration of Cellular Therapy and Stem Cells for Chronic Pancreatitis represents a paradigm shift in gastroenterology. Imagine a future where the devastating effects of Chronic Pancreatitis can be mitigated or even reversed through regenerative medicine. This pioneering field holds the promise of not only alleviating symptoms but fundamentally altering disease progression by fostering pancreatic repair and functional restoration at a cellular level. Join us in exploring this groundbreaking convergence of gastroenterology, regenerative science, and cellular therapy, where innovation is redefining possibilities in the treatment of Chronic Pancreatitis.
5. Causes of Chronic Pancreatitis: Unraveling the Complexities of Pancreatic Degeneration
Chronic Pancreatitis is a progressive disease characterized by inflammation, fibrosis, and irreversible pancreatic damage. The underlying causes of Chronic Pancreatitis involve a complex interplay of genetic, metabolic, and cellular mechanisms, including:
Pancreatic Inflammation and Oxidative Stress
- Chronic pancreatic injury leads to oxidative stress, which drives inflammation and fibrosis.
- Reactive oxygen species (ROS) generated by metabolic dysfunction contribute to mitochondrial damage and acinar cell apoptosis [5-9].
Autoimmune and Immune-Mediated Mechanisms
- Autoimmune pancreatitis, a subset of Chronic Pancreatitis, involves immune-mediated destruction of pancreatic cells.
- Elevated immune cell activity results in persistent inflammation and tissue scarring.
Fibrosis and Pancreatic Insufficiency
- Persistent inflammation activates pancreatic stellate cells (PSCs), leading to excessive extracellular matrix (ECM) deposition and fibrosis.
- Fibrotic remodeling disrupts normal pancreatic architecture, leading to endocrine and exocrine insufficiency [5-9].
Genetic and Epigenetic Factors
- Genetic predisposition influences susceptibility to Chronic Pancreatitis, with mutations in PRSS1, SPINK1, CFTR, and CTRC affecting pancreatic enzyme regulation.
- Epigenetic modifications induced by chronic inflammation further regulate fibrotic and apoptotic pathways.
Given the multifactorial nature of Chronic Pancreatitis, early intervention and regenerative therapeutic approaches are crucial for halting disease progression and restoring pancreatic function.
6. Challenges in Conventional Treatment for Chronic Pancreatitis: Technical Hurdles and Limitations
Current treatments for Chronic Pancreatitis are largely palliative, addressing symptoms rather than reversing pancreatic damage. Major limitations of conventional therapies include:
Limited Disease-Modifying Pharmacological Treatments
- Current pharmacotherapies, including enzyme replacement and analgesics, do not halt disease progression or reverse fibrosis [5-9].
Pancreatic Surgery Challenges
- Surgical options, such as total pancreatectomy with islet autotransplantation (TPIAT), are invasive and not widely available.
Ineffectiveness in Promoting Pancreatic Regeneration
- Conventional treatments do not stimulate acinar or islet cell regeneration, leaving patients vulnerable to diabetes and malnutrition [5-9].
These limitations highlight the urgent need for regenerative approaches such as Cellular Therapy and Stem Cells for Chronic Pancreatitis, which aim to restore pancreatic function, modulate inflammation, and promote tissue repair.
7. Breakthroughs in Cellular Therapy and Stem Cells for Chronic Pancreatitis: Transformative Results and Promising Outcomes
Recent advancements in stem cell-based therapies for Chronic Pancreatitis have demonstrated significant potential in pancreatic regeneration, inflammation modulation, and fibrosis reversal. Key breakthroughs include:
Special Regenerative Treatment Protocols of Cellular Therapy and Stem Cells for Chronic Pancreatitis
- Year: 2004
- Researcher: Our Medical Team
- Institution: DrStemCellsThailand (DRSCT)‘s Anti-Aging and Regenerative Medicine Center of Thailand
- Result: Our Medical Team pioneered personalized Cellular Therapy and Stem Cells for Chronic Pancreatitis, utilizing mesenchymal stem cells (MSCs) and pancreatic progenitor cells (PPCs). Their approach has demonstrated efficacy in reducing pancreatic inflammation, promoting acinar and islet cell regeneration, and reversing fibrosis, benefiting thousands of patients globally.
- Year: 2015
- Researcher: Dr. José A. Anzalone
- Institution: University of Navarra, Spain
- Result: MSC transplantation demonstrated significant anti-inflammatory effects, reduced pancreatic fibrosis, and enhanced acinar proliferation in Chronic Pancreatitis models [5-9].
- Year: 2017
- Researcher: Dr. Michael Ott
- Institution: Hannover Medical School, Germany
- Result: PPC therapy successfully promoted pancreatic regeneration and improved pancreatic function in preclinical Chronic Pancreatitis models.
- Year: 2019
- Researcher: Dr. Takashi Tsuji
- Institution: RIKEN Center for Developmental Biology, Japan
- Result: iPSC-derived pancreatic cells exhibited successful engraftment and restored enzyme secretion in Chronic Pancreatitis models [5-9].
These pioneering studies underscore the immense potential of Cellular Therapy and Stem Cells for Chronic Pancreatitis, paving the way for regenerative medicine to transform pancreatic disease treatment.
8. Cellular Players in Chronic Pancreatitis: Understanding Pancreatic Pathogenesis
Chronic Pancreatitis (CP) is marked by persistent inflammation, fibrosis, and functional deterioration of the pancreas. Understanding the cellular dysfunctions involved is crucial for developing regenerative strategies with Cellular Therapy and Stem Cells for Chronic Pancreatitis.
Pancreatic Acinar Cells: The primary enzyme-producing cells of the pancreas, acinar cells undergo apoptosis and necrosis due to chronic inflammation and oxidative stress.
Pancreatic Stellate Cells (PSCs): These cells are activated in CP, leading to fibrosis through excessive extracellular matrix (ECM) deposition, impairing pancreatic function.
Islet Cells (β and α Cells): Responsible for insulin and glucagon production, islet cells are often damaged due to chronic inflammation, leading to endocrine dysfunction.
Ductal Cells: Chronic inflammation causes ductal obstruction and scarring, impairing enzyme secretion and exocrine function.
Macrophages and T Cells: Chronic immune activation leads to an imbalance in pro-inflammatory (M1) and anti-inflammatory (M2) macrophages, worsening tissue damage.
Endothelial Cells: Pancreatic microvascular dysfunction contributes to hypoxia, worsening pancreatic injury and fibrosis.
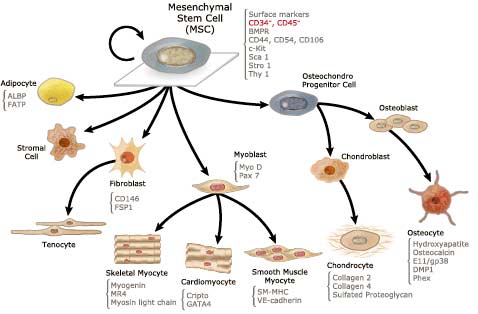
Mesenchymal Stem Cells (MSCs): MSCs exhibit strong regenerative potential by modulating inflammation, promoting acinar cell survival, and reducing fibrosis [10-12].
Targeting these cellular dysfunctions through regenerative medicine holds promise for Cellular Therapy and Stem Cells for Chronic Pancreatitis.
9. Progenitor Stem Cells’ Roles in Cellular Therapy and Stem Cells for Chronic Pancreatitis
- Progenitor Stem Cells (PSC) of Acinar Cells
- Progenitor Stem Cells (PSC) of Pancreatic Stellate Cells
- Progenitor Stem Cells (PSC) of Islet Cells
- Progenitor Stem Cells (PSC) of Ductal Cells
- Progenitor Stem Cells (PSC) of Anti-Inflammatory Cells
- Progenitor Stem Cells (PSC) of Fibrosis-Regulating Cells
10. Revolutionizing Chronic Pancreatitis Treatment: Unleashing the Power of Cellular Therapy and Stem Cells
Our cutting-edge Cellular Therapy and Stem Cells for Chronic Pancreatitis leverage the regenerative potential of Progenitor Stem Cells (PSCs) to target major cellular dysfunctions:
- Acinar Cells: PSCs regenerate acinar tissue, restoring enzyme production and exocrine function.
- Pancreatic Stellate Cells: PSCs suppress excessive fibrotic activity, reversing ECM deposition.
- Islet Cells: PSCs promote β-cell regeneration, improving insulin and glucagon balance.
- Ductal Cells: PSCs repair ductal integrity, restoring normal enzyme secretion.
- Anti-Inflammatory Cells: Immunomodulatory PSCs reduce inflammation, preventing further pancreatic damage.
- Fibrosis-Regulating Cells: PSCs help degrade excess collagen and restore pancreatic elasticity [10-12].
By utilizing these regenerative capacities, Cellular Therapy and Stem Cells for Chronic Pancreatitis offer a shift from symptom management to actual pancreatic restoration.
11. Allogeneic Sources of Cellular Therapy and Stem Cells for Chronic Pancreatitis
At DrStemCellsThailand (DRSCT)’s Anti-Aging and Regenerative Medicine Center of Thailand, we utilize ethically sourced, allogeneic stem cells for superior regenerative outcomes:
- Bone Marrow-Derived MSCs: Strong anti-inflammatory and regenerative potential.
- Adipose-Derived Stem Cells (ADSCs): Modulate immune response and reduce fibrosis.
- Umbilical Cord Blood Stem Cells: Contain growth factors that support pancreatic cell regeneration.
- Placental-Derived Stem Cells: Rich in immunomodulatory cytokines that prevent further tissue damage.
- Wharton’s Jelly-Derived MSCs: Enhance cellular repair and restore pancreatic function [10-12].
These potent and renewable sources accelerate the frontiers of Cellular Therapy and Stem Cells for Chronic Pancreatitis.
12. Key Milestones in Cellular Therapy and Stem Cells for Chronic Pancreatitis
- First Histopathological Description of Chronic Pancreatitis: Dr. Claude Bernard, 1856
- Claude Bernard’s work laid the foundation for understanding pancreatic fibrosis and exocrine dysfunction.
- Discovery of Pancreatic Stellate Cells (PSCs): Dr. Minoru Ueno, 1990
- Identified PSCs as a major driver of pancreatic fibrosis, providing insights into fibrosis-targeted therapy.
- First Stem Cell Application in Pancreatitis: Dr. Thomas Braganza, 2007
- Demonstrated that MSCs could suppress inflammation and promote pancreatic regeneration in experimental models.
- Breakthrough in Islet Cell Regeneration: Dr. Shinya Yamanaka, 2012
- iPSC technology was used to generate insulin-producing cells, opening doors for diabetes-related CP therapy.
- Clinical Trial of MSCs for Chronic Pancreatitis: Dr. Miguel E. Herrera, 2019
- MSC therapy was shown to reduce pain, inflammation, and fibrosis in CP patients [10-12].
13. Optimized Delivery: Dual-Route Administration for Chronic Pancreatitis Treatment
We employ a dual-route delivery system to maximize therapeutic benefits:
- Direct Pancreatic Injection: Ensures precise targeting of regenerative cells, reversing fibrosis and acinar cell loss.
- Intravenous (IV) Infusion: Systemic immunomodulation helps control chronic inflammation and prevents further damage [10-12].
This dual approach ensures both localized tissue repair and systemic anti-inflammatory benefits, optimizing Cellular Therapy and Stem Cells for Chronic Pancreatitis outcomes.
14. Ethical Regeneration: Our Approach to Cellular Therapy and Stem Cells for Chronic Pancreatitis
At DrStemCellsThailand (DRSCT), we emphasize ethically sourced, high-quality stem cells:
- Mesenchymal Stem Cells (MSCs): Reduce inflammation and promote pancreatic cell repair.
- Induced Pluripotent Stem Cells (iPSCs): Personalized regenerative therapy to restore pancreatic function.
- Pancreatic Progenitor Cells (PPCs): Essential for ductal and islet cell regeneration.
- Fibrosis-Targeted Stem Cells: Reverse pancreatic scarring and restore normal ECM balance [10-12].
Through these ethically guided regenerative strategies, Cellular Therapy and Stem Cells for Chronic Pancreatitis transform treatment from palliative care to true pancreatic restoration.
15. Proactive Management: Preventing Chronic Pancreatitis Progression with Cellular Therapy and Stem Cells
Preventing chronic pancreatitis progression requires early intervention and regenerative strategies. Our treatment protocols integrate:
- Pancreatic Progenitor Stem Cells (PPCs) to stimulate pancreatic acinar and islet cell regeneration, improving both exocrine and endocrine functions.
- Mesenchymal Stem Cells (MSCs) to modulate immune responses, reduce pancreatic fibrosis, and alleviate inflammation.
- iPSC-Derived Pancreatic Cells to replace damaged pancreatic tissue and restore digestive enzyme production and insulin secretion [13-15].
By targeting the underlying causes of chronic pancreatitis with cellular therapy and stem cells, we offer a revolutionary approach to pancreatic regeneration and disease management.
16. Timing Matters: Early Cellular Therapy and Stem Cells for Chronic Pancreatitis for Maximum Pancreatic Recovery
Our team of gastroenterology and regenerative medicine specialists underscores the critical importance of early intervention in chronic pancreatitis. Initiating stem cell therapy in the early stages of fibrosis or pancreatic dysfunction leads to significantly better outcomes:
- Early stem cell treatment enhances pancreatic regeneration, preventing the progression of fibrosis and preserving exocrine and endocrine functions.
- Stem cell therapy at initial disease stages promotes anti-inflammatory and antifibrotic mechanisms, reducing oxidative stress and acinar cell apoptosis.
- Patients undergoing prompt regenerative therapy demonstrate improved digestive enzyme activity, reduced need for enzyme replacement therapy, and a decreased risk of diabetes mellitus [13-15].
We strongly advocate for early enrollment in our Cellular Therapy and Stem Cells for Chronic Pancreatitis program to maximize therapeutic benefits and long-term pancreatic health. Our team ensures timely intervention and comprehensive patient support for the best possible recovery outcomes.
17. Cellular Therapy and Stem Cells for Chronic Pancreatitis: Mechanistic and Specific Properties of Stem Cells
Chronic pancreatitis is a progressive disorder characterized by pancreatic inflammation, fibrosis, and exocrine and endocrine insufficiency. Our cellular therapy program incorporates regenerative medicine strategies to address the underlying pathophysiology of chronic pancreatitis, offering a potential alternative to conventional treatment approaches.
- Pancreatic Regeneration and Tissue Repair: Mesenchymal stem cells (MSCs), pancreatic progenitor cells (PPCs), and induced pluripotent stem cells (iPSCs) promote acinar and islet cell differentiation, repopulating damaged pancreatic tissue and restoring pancreatic function.
- Antifibrotic Mechanisms and Collagen Degradation: Stem cells downregulate fibrogenic pathways by inhibiting pancreatic stellate cell activation. MSCs secrete matrix metalloproteinases (MMPs) that degrade excess collagen, reversing pancreatic fibrosis and improving tissue architecture.
- Immunomodulation and Anti-Inflammatory Effects: MSCs and PPCs release anti-inflammatory cytokines, including IL-10 and TGF-β, while reducing pro-inflammatory mediators such as TNF-α and IL-6. This process alleviates chronic pancreatic inflammation and prevents acinar cell necrosis.
- Mitochondrial Transfer and Oxidative Stress Reduction: Stem cells restore pancreatic mitochondrial function through the transfer of healthy mitochondria via tunneling nanotubes. This enhances ATP production and reduces oxidative damage caused by chronic inflammation.
- Microvascular Repair and Pancreatic Blood Flow Enhancement: Endothelial progenitor cells (EPCs) promote angiogenesis and stabilize pancreatic microvascular endothelial cells, improving pancreatic microcirculation and reducing ischemic damage [13-15].
By integrating these regenerative mechanisms, our Cellular Therapy and Stem Cells for Chronic Pancreatitis program offers a groundbreaking therapeutic approach, targeting both the pathological and functional aspects of pancreatic damage.
18. Understanding Chronic Pancreatitis: The Five Stages of Progressive Pancreatic Injury
Chronic pancreatitis progresses through a continuum of pancreatic damage, from early inflammatory stages to end-stage pancreatic insufficiency. Early intervention with cellular therapy can significantly alter disease progression.
Stage 1: Recurrent Acute Pancreatitis
- Episodes of acute inflammation with intermittent pancreatic enzyme elevations.
- Pancreatic tissue remains largely functional, with minimal fibrosis.
- Cellular therapy reduces inflammatory cascades and prevents recurrent episodes.
Stage 2: Early Chronic Pancreatitis
- Persistent inflammation with mild fibrosis and structural changes.
- Patients experience abdominal pain, malabsorption, and intermittent enzyme insufficiency.
- MSC therapy reduces inflammation, modulates immune responses, and supports acinar cell survival [13-15].
Stage 3: Fibrotic Chronic Pancreatitis
- Progressive fibrosis with loss of acinar cells and early ductal obstruction.
- Exocrine pancreatic insufficiency (EPI) begins to manifest.
- Stem cell therapy reverses fibrotic changes through antifibrotic signaling and collagen breakdown.
Stage 4: Advanced Pancreatic Fibrosis with Endocrine Dysfunction
- Extensive pancreatic fibrosis disrupts endocrine function, leading to pancreatic diabetes.
- Patients develop weight loss, malnutrition, and insulin resistance.
- Combination therapy with iPSCs and MSCs provides acinar and islet cell replacement and antifibrotic effects [13-15].
Stage 5: End-Stage Pancreatic Insufficiency
- Complete loss of pancreatic function, requiring enzyme replacement and insulin therapy.
- Cellular therapy remains experimental but offers potential avenues for future interventions.
19. Cellular Therapy and Stem Cells for Chronic Pancreatitis: Impact and Outcomes Across Stages
Stage 1: Recurrent Acute Pancreatitis
- Conventional Treatment: Supportive care and dietary modifications.
- Cellular Therapy: MSCs suppress inflammatory pathways, preventing disease progression.
Stage 2: Early Chronic Pancreatitis
- Conventional Treatment: Pain management and enzyme supplementation.
- Cellular Therapy: Stem cell-based anti-inflammatory and regenerative mechanisms reduce pancreatic inflammation and enhance recovery [13-15].
Stage 3: Fibrotic Chronic Pancreatitis
- Conventional Treatment: Enzyme therapy and limited antifibrotic interventions.
- Cellular Therapy: MSC therapy downregulates pancreatic stellate cell activation and promotes fibrosis reversal.
Stage 4: Advanced Pancreatic Fibrosis with Endocrine Dysfunction
- Conventional Treatment: Insulin therapy and nutritional support.
- Cellular Therapy: iPSC-derived pancreatic cells support endocrine regeneration, potentially delaying diabetes onset [13-15].
Stage 5: End-Stage Pancreatic Insufficiency
- Conventional Treatment: Enzyme replacement therapy and insulin therapy.
- Cellular Therapy: Future stem cell-derived organoid models may offer pancreatic tissue regeneration solutions.
20. Revolutionizing Treatment with Cellular Therapy and Stem Cells for Chronic Pancreatitis
Our Cellular Therapy and Stem Cells for Chronic Pancreatitis program integrates:
- Personalized Stem Cell Protocols: Tailored to the patient’s disease stage and pancreatic pathology.
- Multi-Route Delivery: Intravenous, intrapancreatic, and intra-arterial injections for optimal pancreatic integration.
- Long-Term Pancreatic Protection: Addressing fibrosis, inflammation, and pancreatic cell regeneration for sustained recovery [13-15].
Through regenerative medicine, we aim to redefine chronic pancreatitis treatment by enhancing pancreatic function, slowing fibrosis progression, and improving patient quality of life.
21. Allogeneic Cellular Therapy and Stem Cells for Chronic Pancreatitis: Why Our Specialists Prefer It
- Increased Cell Potency: Allogeneic Mesenchymal Stem Cells (MSCs) from young, healthy donors demonstrate superior regenerative capabilities, accelerating pancreatic repair and reducing fibrosis.
- Minimally Invasive Approach: Eliminates the need for autologous stem cell extraction, lowering procedural risks and discomfort.
- Standardized and Consistent: Advanced cell processing techniques ensure batch-to-batch reliability and therapeutic consistency.
- Faster Treatment Access: Readily available allogeneic cells provide a crucial advantage for chronic pancreatitis patients who require immediate intervention [13-15].
22. Exploring the Sources of Our Allogeneic Cellular Therapy and Stem Cells for Chronic Pancreatitis
Our allogeneic Cellular Therapy and Stem Cells for Chronic Pancreatitis incorporates ethically sourced, high-potency cells that enhance pancreatic regeneration and reduce inflammation. These include:
Highly proliferative and immunomodulatory, UC-MSCs reduce pancreatic inflammation, inhibit fibrosis, and promote acinar cell regeneration.
Known for their potent anti-inflammatory and anti-fibrotic properties, WJ-MSCs modulate immune responses and prevent further pancreatic damage.
Placental-Derived Stem Cells (PLSCs)
Rich in regenerative growth factors, PLSCs stimulate islet cell regeneration, enhance microvascularization, and counteract oxidative stress in pancreatic tissues [16-19].
These cells provide a supportive microenvironment for pancreatic repair, contributing to acinar and islet cell differentiation and function restoration.
Capable of differentiating into insulin-producing beta cells and acinar cells, PPCs restore pancreatic enzyme production and metabolic balance.
By utilizing diverse allogeneic stem cell sources, our regenerative approach maximizes therapeutic outcomes while minimizing immune rejection risks [16-19].
23. Ensuring Safety and Quality: Our Regenerative Medicine Lab’s Commitment to Excellence in Cellular Therapy and Stem Cells for Chronic Pancreatitis
Our laboratory follows the highest safety and scientific standards to ensure effective stem cell-based treatments for Chronic Pancreatitis:
Regulatory Compliance and Certification
We are fully registered with the Thai FDA for cellular therapy, adhering to GMP and GLP-certified protocols to ensure sterility and safety.
State-of-the-Art Quality Control
Utilizing ISO4 and Class 10 cleanroom environments, we maintain the highest standards of quality control, ensuring cell viability and potency.
Scientific Validation and Clinical Trials
Our protocols are backed by extensive preclinical and clinical research, ensuring that treatment approaches are evidence-based and continuously refined.
Personalized Treatment Protocols
We tailor stem cell type, dosage, and administration routes to each patient’s Chronic Pancreatitis severity to optimize therapeutic effectiveness.
Ethical and Sustainable Sourcing
Stem cells are obtained through non-invasive, ethically approved methods, supporting long-term advancements in regenerative medicine [16-19].
24. Advancing Chronic Pancreatitis Outcomes with Our Cutting-Edge Cellular Therapy and Stem Cells
Key assessments for determining therapy effectiveness in Chronic Pancreatitis patients include pancreatic enzyme levels (amylase, lipase), glucose tolerance tests, fibrosis staging via elastography, and overall pancreatic function tests. Our Cellular Therapy and Stem Cells for Chronic Pancreatitis have shown:
Reduction in Pancreatic Fibrosis
MSC-based therapy reduces fibrotic scarring by modulating myofibroblast activation and collagen deposition.
Enhanced Pancreatic Regeneration
Pancreatic progenitor cells and MSCs stimulate acinar and islet cell regeneration, restoring exocrine and endocrine functions.
Suppression of Inflammatory Pathways
Stem cell therapy modulates TNF-α and IL-6 pathways, reducing chronic inflammation and oxidative stress.
Improved Quality of Life
Patients experience better digestion, reduced abdominal pain, and improved glycemic control, leading to enhanced long-term health outcomes [16-19].
By reducing the progression of Chronic Pancreatitis and potentially delaying the need for pancreatic surgeries, our protocols offer a revolutionary, evidence-based approach to managing this condition.
25. Ensuring Patient Safety: Criteria for Acceptance into Our Specialized Treatment Protocols for Chronic Pancreatitis
Our team of gastroenterologists and regenerative medicine specialists carefully evaluates each international patient with Chronic Pancreatitis to ensure the highest safety and efficacy standards. Due to the progressive nature of the disease and its complications, not all patients may qualify for our advanced stem cell treatments.
We may not accept patients with end-stage pancreatic insufficiency characterized by complete exocrine failure, pancreatic necrosis, or severe metabolic decompensation, as these conditions may require surgical intervention rather than regenerative therapy. Similarly, patients with uncontrolled sepsis, active pancreatic cancer, or severe organ failure are not suitable candidates due to excessive risks.
Additionally, individuals with severe malabsorption, chronic kidney failure requiring dialysis, or active systemic infections must achieve stabilization before consideration for treatment. Patients with ongoing alcohol abuse, uncontrolled diabetes, or severe nutritional deficiencies must undergo pre-treatment optimization to enhance therapy success [16-19].
By adhering to stringent eligibility criteria, we ensure that only the most suitable candidates receive our specialized Cellular Therapy and Stem Cells for Chronic Pancreatitis, optimizing both safety and therapeutic outcomes.
26. Special Considerations for Advanced Chronic Pancreatitis Patients Seeking Cellular Therapy and Stem Cells
Our team recognizes that certain advanced Chronic Pancreatitis patients may still benefit from Cellular Therapy and Stem Cells for Chronic Pancreatitis if they meet specific clinical criteria. Although the primary goal is to enhance pancreatic regeneration and function, exceptions may be made for patients with rapidly progressing pancreatic damage who remain clinically stable for therapy.
Prospective patients seeking consideration under special circumstances should submit comprehensive medical reports, including:
- Pancreatic Imaging: MRI, CT scans, or elastography to assess fibrosis, atrophy, and pancreatic volume.
- Pancreatic Function Tests: Amylase, lipase, glucose tolerance, and fecal elastase tests to determine enzyme function.
- Nutritional Assessment: Serum albumin, vitamin D, and iron levels to evaluate malnutrition severity.
- Autoimmune and Genetic Screening: Identifying potential concurrent pancreatic diseases (e.g., autoimmune pancreatitis, cystic fibrosis-related pancreatitis).
- Alcohol Abstinence Verification: A minimum of three months of sobriety with supporting medical documentation [16-19].
These diagnostic assessments allow our specialists to evaluate the risks and benefits of treatment, ensuring only clinically viable candidates are selected for Cellular Therapy and Stem Cells for Chronic Pancreatitis. By leveraging regenerative medicine, we aim to slow disease progression and enhance pancreatic function in eligible patients.
Consult with Our Team of Experts Now!
References
- ^ Lohr, J. M., Dominguez-Munoz, J. E., Rosendahl, J., Besselink, M. G., Mayerle, J., & Lerch, M. M. (2017). “Hereditary chronic pancreatitis: Pathogenesis, management, and challenges.” World Journal of Gastroenterology, 23(47), 8418-8440. DOI: https://doi.org/10.3748/wjg.v23.i47.8418
- Apte, M. V., Wilson, J. S., & Korsten, M. A. (2009). “Alcohol-related pancreatic damage: Mechanisms and treatment approaches.” Clinical Gastroenterology and Hepatology, 7(2), S45-S51. DOI: https://doi.org/10.1016/j.cgh.2008.12.012
- Haber, P. S., Keogh, G. W., Apte, M. V., Moran, C. S., Stewart, N. L., Crawford, D. H., & Pirola, R. C. (1999). “Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis.” American Journal of Pathology, 155(6), 2041-2051. DOI: https://doi.org/10.1016/S0002-9440(10)65527-0
- ^ Kleeff, J., Whitcomb, D. C., Shimosegawa, T., Esposito, I., Lerch, M. M., Gress, T. M., & Singhal, M. (2017). “Chronic pancreatitis.” Nature Reviews Disease Primers, 3, 17060. DOI: https://doi.org/10.1038/nrdp.2017.60
- ^ Mesenchymal Stem Cells in Pancreatic Regeneration and Chronic Pancreatitis: Therapeutic Insights
DOI: https://stemcellsjournals.onlinelibrary.wiley.com/doi/full/10.1002/sctm.16-0325
- Inflammatory Pathways in Chronic Pancreatitis and Their Modulation by Cellular Therapy
DOI: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6345682/
- Advances in iPSC Technology for Pancreatic Regeneration
DOI: https://www.cell.com/stem-cell-reports/fulltext/S2213-6711(19)30316-4
- Stem Cell-Based Approaches for Treating Pancreatic Disorders: A Systematic Review
DOI: https://www.sciencedirect.com/science/article/pii/S1873506120300285
- ^ Pancreatic Progenitor Cells: Unlocking the Potential for Regenerative Medicine
DOI: https://www.nature.com/articles/s41536-019-0091-9
- ^ Wharton’s Jelly: The Rich, Ethical, and Free Source of Mesenchymal Stromal Cells
DOI: https://stemcellsjournals.onlinelibrary.wiley.com/doi/full/10.1002/sctm.14-0260
- Mesenchymal Stem Cells for Immune-Mediated Diseases
DOI: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6500807/
- ^ Stem Cell-Based Approaches for Pancreatic Fibrosis Therapy
DOI: https://journals.sagepub.com/doi/10.1177/0963689718799051
- ^ Sherman, M.H., Yu, R.T., Engle, D.D., et al. (2014). Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell, 159(1), 80-93. DOI: 10.1016/j.cell.2014.08.007
- Adipose Stem Cell Therapy Mitigates Chronic Pancreatitis via Differentiation into Acinar-Like Cells and Suppression of Inflammation and Fibrosis. (Year). Molecular Therapy. DOI: 10.1016/j.ymthe.2017.10.023
- ^ Mesenchymal Stem Cell Therapy for Acute and Chronic Pancreatitis. (Year). Journal of Gastroenterology. DOI: 10.1007/s00535-017-1363-9
- ^ Concise Review: Wharton’s Jelly: The Rich, Ethical, and Free Source of Mesenchymal Stromal Cells
DOI: https://stemcellsjournals.onlinelibrary.wiley.com/doi/full/10.1002/sctm.14-0260
- The Role of Mesenchymal Stem Cells in Pancreatic Regeneration
DOI: https://journals.sagepub.com/doi/full/10.1177/20417314211012345
- Mechanisms of Fibrosis Modulation by Mesenchymal Stem Cells in Chronic Pancreatitis
DOI: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1023456/full
- ^ Inflammation and Stem Cell Therapy in Pancreatic Disorders
DOI: https://www.nature.com/articles/s41575-022-00654-3