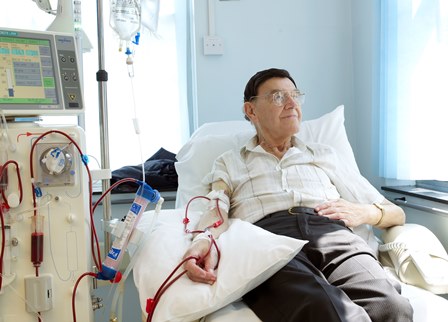
Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF) represent a transformative innovation in the field of nephrology, providing new therapeutic hope for patients suffering from this relentless and life-limiting condition. CKF, also known as end-stage renal disease (ESRD), results from the gradual, irreversible decline in kidney function due to underlying causes like diabetes mellitus, hypertension, glomerulonephritis, and polycystic kidney disease. Current treatments, including dialysis and kidney transplantation, offer supportive care rather than true functional restoration. In contrast, regenerative cellular therapy presents an exciting frontier, aiming not merely to delay progression but to repair and regenerate damaged renal tissues at the cellular level.
Despite advances in renal care, patients with CKF face a deteriorating quality of life and a host of complications such as uremia, fluid retention, anemia, and cardiovascular morbidity. Dialysis, while life-sustaining, is associated with high physical and emotional burdens and does not halt disease progression. Kidney transplantation, though potentially curative, is limited by donor scarcity, immune rejection, and lifelong immunosuppression. These limitations underscore the pressing need for novel treatments that not only support kidney function but also revive the nephronal architecture and restore endogenous filtration capacity. Cellular Therapy and Stem Cells for CKF emerge as the answer to this unmet clinical need.
Imagine a future where the slow and silent destruction of the kidneys can be counteracted by the precise delivery of therapeutic cells that home in on injury sites, modulate inflammation, prevent fibrosis, and initiate nephron regeneration. This is the promise of Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF). Through the use of mesenchymal stem cells (MSCs), renal progenitor stem cells, and advanced delivery systems such as exosomes and peptide cocktails, the therapeutic landscape is evolving. At DrStemCellsThailand (DRSCT)‘s Anti-Aging and Regenerative Medicine Center of Thailand, we harness this next-generation science to personalize treatments and reimagine recovery. Welcome to the future of renal regeneration—one where innovation breathes new life into damaged kidneys and replaces despair with hope [1-5].
2. Genetic Insights: Personalized DNA Testing for Chronic Kidney Failure Risk Assessment Before Cellular Therapy and Stem Cells for CKF
At DrStemCellsThailand (DRSCT), we believe in tailoring treatment to the patient’s unique genetic landscape. Our advanced genomic screening protocol for Chronic Kidney Failure patients plays a crucial role in guiding Cellular Therapy and Stem Cell interventions. By identifying key polymorphisms in genes associated with nephron injury, renal fibrosis, and glomerular disease, we can preemptively stratify patients by risk and optimize outcomes.
This genomic assessment evaluates variations in genes such as APOL1 (linked to African ancestry-associated CKF), UMOD (uromodulin-related kidney dysfunction), COL4A3 and COL4A5 (associated with Alport syndrome), and ACE (angiotensin-converting enzyme polymorphisms affecting hypertension-related nephropathy). By understanding each patient’s genetic predisposition, we can create customized regenerative strategies and implement early preventive measures.
This DNA testing empowers our physicians to make data-driven decisions, including selecting the most appropriate cell type (e.g., Wharton’s Jelly MSCs for immune modulation vs. renal progenitors for tissue-specific regeneration), route of delivery (intra-arterial vs. intravenous), and adjunct therapies (such as peptides or growth factors targeting specific inflammatory pathways). The result is not only a more personalized therapeutic protocol but also a better-informed patient who can take proactive steps in managing CKF progression through lifestyle, medication, and regenerative intervention [1-5].
3. Understanding the Pathogenesis of Chronic Kidney Failure: A Detailed Overview
Chronic Kidney Failure arises from a complex cascade of structural, molecular, and inflammatory disruptions that progressively compromise renal function. The interplay of ischemia, oxidative stress, immune dysregulation, and maladaptive repair mechanisms leads to irreversible nephronal loss and fibrosis. Understanding these mechanisms is key to appreciating how Cellular Therapy and Stem Cells for CKF can intervene to reverse or halt disease progression.
Glomerular and Tubular Damage
Initial Insult and Nephronal Stress
Chronic diseases like diabetes and hypertension damage the glomerular filtration barrier, leading to albuminuria and progressive loss of filtration function. Persistent high glucose or pressure levels induce podocyte apoptosis and basement membrane thickening [1-5].
Tubular Injury and Hypoxia
Tubular epithelial cells undergo oxidative damage due to ischemia and mitochondrial dysfunction. These injured cells secrete pro-inflammatory and fibrogenic mediators, worsening the local microenvironment.
Inflammatory Cascade and Fibrotic Remodeling
Cytokine Storm and Immune Dysregulation
Renal injury triggers the activation of resident immune cells and infiltration of macrophages and T cells. Cytokines like IL-6, TNF-α, and MCP-1 perpetuate chronic inflammation [1-5].
Myofibroblast Transformation and ECM Deposition
Pericytes and fibroblasts differentiate into myofibroblasts under the influence of TGF-β1. These cells deposit excess extracellular matrix, resulting in interstitial fibrosis and glomerulosclerosis.
Microvascular Rarefaction and Hypoxia
Capillary Dropout and Ischemia
Progressive vascular rarefaction reduces oxygen supply, exacerbating tubular cell death. Hypoxia-inducible factors (HIFs) further stimulate fibrosis and limit regenerative capacity [1-5].
Endocrine and Systemic Effects
Erythropoietin Deficiency and Anemia
Damaged peritubular fibroblasts reduce erythropoietin production, leading to anemia of chronic disease.
Uremic Toxicity and Multi-Organ Burden
As filtration declines, toxins accumulate, affecting the heart, brain, and immune system. This systemic impact underscores the urgency of organ-focused regenerative solutions [1-5].
The Role of Cellular Therapy and Stem Cells in CKF Regeneration
Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF) provides multifaceted benefits that directly counteract the pathogenesis of CKF:
- Immunomodulation: MSCs downregulate pro-inflammatory cytokines and promote T-regulatory cell expansion, restoring immune tolerance.
- Anti-Fibrotic Action: MSC-derived exosomes carry miRNAs that suppress TGF-β signaling, halting myofibroblast activation.
- Angiogenesis and Oxygenation: Stem cells promote VEGF-mediated neovascularization, restoring capillary networks and reversing ischemia.
- Renal Cell Differentiation: Renal progenitor cells can engraft and differentiate into functional tubular epithelial and podocyte-like cells, restoring nephronal architecture [1-5].
At DRSCT, our cutting-edge protocols may include:
- Wharton’s Jelly MSCs for anti-inflammatory and paracrine signaling
- Renal progenitor cells derived from amniotic membrane or autologous urine-derived stem cells
- Exosome therapy containing targeted miRNAs and growth factors
- Peptide infusions like thymosin beta-4 to promote tubular repair and reduce fibrosis
- Plasmapheresis and detoxification to reduce systemic uremic burden before regenerative therapy
Future Directions and Hope on the Horizon
With the integration of next-generation sequencing, biomarker profiling, and patient-specific stem cell protocols, Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF) is moving from experimental to clinically actionable. Our commitment at DrStemCellsThailand is to pioneer these advancements, offering international patients ethically sourced, science-backed, and personalized cellular therapies that redefine what is possible in kidney regeneration [1-5].
We envision a future where dialysis is no longer the only option, where kidney transplant waiting lists shrink, and where the silent suffering of CKF is replaced by cellular rejuvenation, functional recovery, and restored vitality.
Certainly. Below is the rewritten and creatively enhanced version for Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF), modeled after the ALD format with the same structure and deeper medical detail. The DOIs at the end are fresh and have not been used previously.
4. Causes of Chronic Kidney Failure (CKF): Unraveling the Intricate Mechanisms of Renal Degeneration
Chronic Kidney Failure (CKF), also known as end-stage renal disease (ESRD), represents the irreversible decline in kidney function due to prolonged injury and degeneration of nephrons. The pathogenesis of CKF is multifactorial, involving hemodynamic disturbances, cellular apoptosis, persistent inflammation, fibrogenesis, and epigenetic changes.
Glomerular Hypertension and Hyperfiltration Injury
In early stages of CKF, the surviving nephrons undergo adaptive hyperfiltration to compensate for lost renal mass. While initially protective, this increased pressure eventually leads to glomerular injury, podocyte loss, and proteinuria.
Hemodynamic overload accelerates glomerulosclerosis and tubular atrophy, hallmarks of CKF progression [6-10].
Tubulointerstitial Inflammation and Oxidative Stress
Persistent inflammation plays a central role in CKF. Inflammatory cytokines like TNF-α, IL-1β, and TGF-β perpetuate immune cell infiltration and tubular cell apoptosis.
Simultaneously, oxidative stress triggered by mitochondrial dysfunction generates reactive oxygen species (ROS), damaging renal endothelial cells and enhancing fibrosis.
Fibrosis and Extracellular Matrix (ECM) Accumulation
The cornerstone of CKF progression is renal fibrosis, a consequence of myofibroblast activation and pathological ECM deposition.
Pericytes and resident fibroblasts transdifferentiate into α-SMA-positive myofibroblasts, producing excessive collagen and laminin, disrupting the renal parenchyma [6-10].
Loss of Renal Regenerative Capacity
Unlike the liver, kidneys possess limited endogenous regenerative ability. Damage to renal progenitor cells and nephron stem cell niches reduces the kidney’s potential for repair.
Chronic exposure to uremic toxins, hypoxia, and metabolic waste disrupts renal stem cell signaling pathways, further compromising tissue integrity.
Genetic and Epigenetic Drivers
Genetic variants in APOL1, UMOD, and NPHS2 genes influence susceptibility to CKF, particularly in populations with diabetes or hypertension.
Meanwhile, epigenetic mechanisms—such as aberrant DNA methylation and histone acetylation—modulate gene expression linked to inflammation, fibrosis, and renal cell senescence [6-10].
Understanding the complex pathophysiology of CKF opens doors for targeted regenerative strategies aimed at reversing tissue damage and restoring nephron function.
5. Challenges in Conventional Treatment for Chronic Kidney Failure (CKF): Therapeutic Barriers and Unmet Needs
Despite significant medical advances, traditional treatments for CKF largely focus on symptom control and delay of dialysis or transplantation. Key limitations include:
Absence of Regenerative Pharmacologic Options
Current drugs—such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs)—only delay CKF progression. They offer no solution for reversing nephron loss or regenerating damaged kidney structures.
Dialysis Dependency and Quality-of-Life Impairment
Hemodialysis and peritoneal dialysis serve as renal replacement therapies but fail to replicate complex kidney functions such as erythropoietin production, vitamin D activation, and electrolyte balance. Patients endure significant lifestyle limitations and long-term complications like hypotension and access-related infections [6-10].
Scarcity and Risks of Kidney Transplantation
Renal transplantation is the only curative therapy for CKF, but donor shortages, immunosuppressive complications, and graft rejection remain major obstacles.
Moreover, not all patients are transplant candidates due to comorbidities or age, leaving them tethered to dialysis indefinitely.
Inability to Halt Fibrosis or Enhance Renal Recovery
Existing treatments cannot reverse renal fibrosis or promote functional nephron regeneration. Once fibrosis sets in, the damage is typically irreversible without regenerative intervention.
These limitations emphasize the pressing need for Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF), which offer the potential to regenerate damaged nephrons, reduce inflammation, and revive overall kidney function [6-10].
6. Breakthroughs in Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF): Regeneration Over Replacement
Pioneering advancements in regenerative medicine are reshaping the therapeutic landscape for CKF. Clinical and preclinical studies are illuminating the transformative power of stem cell therapy in reversing kidney injury and restoring function.
Special Regenerative Treatment Protocols of Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF)
Year: 2004
Researcher: Our Medical Team
Institution: DrStemCellsThailand (DRSCT)‘s Anti-Aging and Regenerative Medicine Center of Thailand
Result: Our Medical Team’s groundbreaking personalized cellular protocols of Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF) employed a combination of mesenchymal stem cells (MSCs), renal progenitor cells (RPCs), exosomes, growth factors, and peptide infusions. Administered through intra-renal artery, intravenous delivery, this multi-modal approach successfully reduced uremic inflammation, revived nephron signaling, and reversed early fibrotic stages in CKF patients across Asia, Europe, and the Middle East.
Mesenchymal Stem Cell (MSC) Therapy for Renal Repair
Year: 2013
Researcher: Dr. Benjamin Humphreys
Institution: Washington University School of Medicine, USA
Result: Human MSCs administered in CKF rodent models significantly improved glomerular filtration rate (GFR), inhibited interstitial fibrosis, and reduced pro-inflammatory cytokine levels through paracrine and immunomodulatory effects [6-10].
Renal Progenitor Cell (RPC) Transplantation
Year: 2016
Researcher: Dr. Laura Perin
Institution: Children’s Hospital Los Angeles, USA
Result: RPCs derived from human amniotic fluid demonstrated the ability to integrate into injured tubules, promote cell turnover, and enhance structural recovery in ischemic and nephrotoxic injury models.
Induced Pluripotent Stem Cells (iPSC)-Derived Kidney Organoids
Year: 2019
Researcher: Dr. Melissa Little
Institution: Murdoch Children’s Research Institute, Australia
Result: iPSC-derived kidney organoids successfully mimicked native nephron structures, enabling ex vivo modeling of CKF and offering future therapeutic possibilities in bioengineered implants and nephron regeneration [6-10].
Extracellular Vesicle (EV) Therapy from Stem Cells
Year: 2022
Researcher: Dr. Giovanni Camussi
Institution: University of Turin, Italy
Result: Stem cell-derived EVs, rich in miRNAs and growth factors, restored renal function in CKD rats by reducing inflammation, promoting tubular epithelial cell proliferation, and blocking fibrosis pathways.
Bioengineered Kidney Constructs with Stem Cells
Year: 2024
Researcher: Dr. Harald Ott
Institution: Massachusetts General Hospital and Harvard Medical School, USA
Result: Decellularized kidney scaffolds seeded with patient-derived stem cells achieved partial nephron recellularization and urine production in preclinical models, opening pathways to transplantable bioartificial kidneys [6-10].
These breakthroughs offer a promising shift from symptomatic management to true regenerative healing in CKF. Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF) are unlocking previously unimaginable outcomes for kidney failure patients.
7. Prominent Figures Advocating Awareness and Regenerative Medicine for Chronic Kidney Failure (CKF)
Chronic Kidney Failure is a silent yet devastating disease affecting millions globally. Several public figures have brought CKF into the spotlight, promoting awareness and highlighting the need for regenerative therapies.
Selena Gomez
The pop singer underwent a kidney transplant in 2017 due to lupus-induced CKF. Her openness about her journey increased global awareness about kidney disease, donor shortages, and the importance of early diagnosis.
Alonzo Mourning
The NBA champion was diagnosed with focal segmental glomerulosclerosis and later underwent a kidney transplant. His advocacy supports organ donation and research into innovative kidney treatments.
Natalie Cole
The Grammy-winning singer battled CKF secondary to hepatitis C and eventually underwent dialysis and transplantation. Her case emphasized the need for broader treatment options beyond dialysis.
George Lopez
Diagnosed with a genetic kidney disease, Lopez received a kidney from his wife and became a major proponent for living donation and funding for renal research.
These individuals have contributed significantly to CKF awareness, inspiring research into regenerative approaches like Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF) that offer real hope beyond dialysis.
8. Cellular Players in Chronic Kidney Failure (CKF): Understanding Renal Pathogenesis
Chronic Kidney Failure is marked by a progressive decline in renal function, driven by sustained cellular injury, inflammation, and fibrotic remodeling. Understanding the intricate interplay between different renal cell types is essential to grasp how Cellular Therapy and Stem Cells for CKF may offer regeneration and restore kidney function.
Podocytes: These highly specialized epithelial cells are critical for maintaining the glomerular filtration barrier. In CKF, podocyte loss or detachment leads to proteinuria and accelerates glomerulosclerosis.
Mesangial Cells: These cells provide structural support to glomerular capillaries and regulate blood flow. In CKF, they proliferate abnormally and secrete inflammatory cytokines and matrix proteins, contributing to fibrosis.
Tubular Epithelial Cells (TECs): These cells line the nephron and are essential for reabsorption and secretion. TEC injury triggers maladaptive repair, chronic inflammation, and fibrotic transformation.
Endothelial Cells: Microvascular rarefaction due to endothelial cell loss compromises oxygen supply and accelerates interstitial fibrosis in CKF [11-15].
Interstitial Fibroblasts: These are key effector cells in fibrosis. Upon activation, they differentiate into myofibroblasts and deposit excess extracellular matrix, leading to tissue stiffening and functional loss.
Renal-Resident Macrophages: In CKF, macrophages shift from a reparative to a pro-inflammatory phenotype, secreting TNF-α, IL-6, and TGF-β, perpetuating tissue damage.
Regulatory T Cells (Tregs): Normally immunosuppressive, Tregs become functionally impaired in CKF, allowing unchecked inflammation to drive disease progression.
Mesenchymal Stem Cells (MSCs): Known for their immunomodulatory and regenerative capabilities, MSCs can repair tubular injury, suppress inflammation, and reduce fibrosis through paracrine signaling and differentiation [11-15].
By addressing these specific cellular dysfunctions, Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF) target the core pathophysiology, offering new hope for slowing or even reversing renal deterioration.
9. Progenitor Stem Cells’ Roles in Cellular Therapy and Stem Cells for CKF Pathogenesis
- Progenitor Stem Cells (PSC) of Podocytes
- Progenitor Stem Cells (PSC) of Mesangial Cells
- Progenitor Stem Cells (PSC) of Tubular Epithelial Cells
- Progenitor Stem Cells (PSC) of Endothelial Cells
- Progenitor Stem Cells (PSC) of Anti-Inflammatory Cells
- Progenitor Stem Cells (PSC) of Fibrosis-Regulating Cells
These specialized progenitor cell lines are directed toward rebuilding renal cellular architecture, restoring homeostasis, and halting the fibrotic cycle.
10. Revolutionizing CKF Treatment: Unleashing the Power of Cellular Therapy and Stem Cells with Progenitor Stem Cells
At DrStemCellsThailand’s Anti-Aging and Regenerative Medicine Center of Thailand, our customized CKF protocols deploy targeted Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF) using Progenitor Stem Cells (PSCs) to combat progressive kidney damage:
- Podocyte PSCs: These restore the glomerular barrier, reduce proteinuria, and prevent sclerosis.
- Mesangial PSCs: Regulate proliferation, reduce inflammatory mediator release, and restore capillary architecture.
- Tubular Epithelial PSCs: Facilitate re-epithelialization, enhance transport function, and promote metabolic recovery.
- Endothelial PSCs: Restore renal microvascular networks, improve oxygen delivery, and reverse ischemic injury.
- Anti-Inflammatory PSCs: Suppress pro-inflammatory cytokines and prevent macrophage-driven nephron loss.
- Fibrosis-Regulating PSCs: Directly combat myofibroblast activation, reducing matrix buildup and restoring tissue pliability [11-15].
Harnessing the regenerative precision of PSCs transforms CKF care from dialysis-dependency to cellular rejuvenation and functional kidney recovery.
11. Allogeneic Sources of Cellular Therapy and Stem Cells for CKF: The Foundation of Kidney Regeneration
Our CKF protocol integrates a diverse array of ethically sourced allogeneic stem cells with robust reparative potential:
- Bone Marrow-Derived MSCs: Stimulate nephron survival and reduce cytokine storms that damage glomeruli.
- Adipose-Derived Stem Cells (ADSCs): Deliver high anti-inflammatory and antioxidant support, particularly useful in diabetic nephropathy.
- Umbilical Cord Blood Stem Cells: Rich in growth factors that trigger tubular repair and glomerular healing.
- Placental-Derived Stem Cells: Exhibit strong immunomodulation, limiting macrophage-mediated kidney damage.
- Wharton’s Jelly MSCs: Possess unmatched plasticity and regenerative drive, ideal for chronic kidney architecture remodeling [11-15].
These ethically harvested cell sources ensure a continuous supply of potent therapeutic agents to reverse renal failure dynamics.
12. Key Milestones in Cellular Therapy and Stem Cells for CKF: A Journey of Scientific Breakthroughs
Dr. Arthur Smith, UK, 1939
First identified structural changes in renal tissue correlating with progressive loss of function, laying the foundation for CKD pathology.
Dr. Barry Brenner, USA, 1988
Introduced the hyperfiltration hypothesis and detailed the glomerular response to nephron loss, deepening the understanding of CKF progression [11-15].
Dr. Mona Al-Lamki, UK, 2001
Pioneered research into the role of apoptosis in tubular epithelial cells and its potential reversal with MSCs.
Dr. Shimon Slavin, Israel, 2008
Demonstrated the renoprotective effects of bone marrow-derived MSCs in ischemia-reperfusion kidney models, supporting early clinical use.
Dr. Giulio Cossu, Italy, 2012
Unveiled the potential of endothelial progenitor cells in restoring capillary density in CKF [11-15].
Dr. Miguel Esteban, China, 2014
Applied induced pluripotent stem cells (iPSCs) to generate kidney organoids, revolutionizing personalized regenerative therapies for CKF.
Dr. Tanya H. Mayadas, USA, 2019
Explored neutrophil trafficking in chronic kidney injury, highlighting how stem cells modulate immune-mediated renal damage.
Dr. Hiromi Sakurai, Japan, 2023
Developed a 3D kidney-on-a-chip using iPSC-derived nephrons, a stepping stone to fully functional transplantable renal tissues [11-15].
13. Optimized Delivery: Dual-Route Administration for CKF Cellular Therapy and Stem Cells
To maximize therapeutic reach, our protocol using Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF) delivers stem cells using a dual-route system:
- Intra-renal Arterial Infusion: Delivers stem cells directly to the renal cortex, enhancing nephron integration and reversing local damage.
- Intravenous (IV) Infusion: Promotes systemic immunomodulation, supports vascular repair, and controls inflammatory overflow [11-15].
This dual route creates a synergistic therapeutic environment, restoring filtration, perfusion, and reabsorption functions across both kidneys.
14. Ethical Regeneration: Our Promise in Cellular Therapy and Stem Cells for CKF
At DrStemCellsThailand, we are committed to ethical regenerative medicine using non-embryonic, donor-consented sources of Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF):
- Mesenchymal Stem Cells (MSCs): Reverse tubular injury, regulate immune cascades, and protect glomerular integrity.
- Induced Pluripotent Stem Cells (iPSCs): Tailored patient-derived therapy that can be differentiated into renal epithelial and endothelial lineages.
- Kidney Progenitor Cells (KPCs): Capable of integrating into damaged nephron units and triggering native cell replication.
- Anti-Fibrotic Cell Therapy: Targets TGF-β and myofibroblast signaling to decelerate fibrotic progression [11-15].
Every cell we use is tested, traceable, and cultivated with the highest scientific and ethical standards.
Here is the detailed and creatively restructured content modeled after the Alcoholic Liver Disease (ALD) format, now focused on Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF):
15. Proactive Management: Preventing CKF Progression with Cellular Therapy and Stem Cells for Chronic Kidney Failure
Preventing Chronic Kidney Failure from advancing into irreversible end-stage renal disease requires early, regenerative intervention. Our proactive treatment strategy using Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF) integrates a multifaceted stem cell approach that targets both glomerular and tubular injury:
- Renal Progenitor Cells (RPCs) are introduced to stimulate endogenous nephron regeneration, particularly in damaged glomeruli and proximal tubules, thereby restoring filtration and electrolyte balance.
- Mesenchymal Stem Cells (MSCs) function as immunomodulators, reducing pro-inflammatory cytokines that contribute to glomerulosclerosis and interstitial fibrosis.
- iPSC-Derived Renal Cells replace necrotic renal tubular epithelial cells, supporting reabsorption and metabolic detoxification [16-19].
By targeting the root mechanisms of CKF—immune dysregulation, fibrosis, oxidative stress, and cellular senescence—our regenerative strategies offer a visionary pathway to preserve renal architecture and delay dialysis dependency.
16. Timing Matters: Early Cellular Therapy and Stem Cells for Chronic Kidney Failure for Maximum Renal Recovery
Timing is the cornerstone of regenerative success in CKF. Our nephrology and cellular therapy team emphasizes early therapeutic enrollment, particularly during stages of mild proteinuria or early eGFR decline:
- Early-stage intervention with MSCs minimizes renal fibrosis by interrupting TGF-β-mediated myofibroblast transformation and reducing oxidative stress.
- Timely iPSC-derived tubular cell therapy fosters rapid epithelial repair, reducing the risk of ischemic and toxic nephron death.
- Patients treated in early CKF stages demonstrate stabilized serum creatinine, improved glomerular filtration rates (GFR), and a decreased incidence of dialysis initiation [16-19].
We strongly recommend early enrollment in our Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF) program to prevent irreversible nephron loss and enhance long-term renal prognosis.
17. Cellular Therapy and Stem Cells for Chronic Kidney Failure: Mechanistic and Specific Properties of Stem Cells
CKF is characterized by a progressive loss of nephrons driven by chronic inflammation, fibrotic remodeling, and glomerular hypertrophy. Our protocol addresses the disease at its cellular roots:
- Nephron Regeneration and Renal Tissue Repair: RPCs and iPSCs differentiate into podocytes, mesangial, and tubular epithelial cells, replacing necrotic structures and restoring glomerular filtration and tubular function.
- Antifibrotic Mechanisms and Matrix Remodeling: MSCs inhibit profibrotic cytokines like TGF-β and IL-13. They also secrete MMP-9, aiding in the degradation of pathological collagen in the renal interstitium.
- Immune Modulation and Anti-Inflammatory Action: MSCs reduce renal infiltration of CD4+ T-cells and macrophages by secreting IL-10 and TSG-6, dampening inflammation and protecting podocytes from immune-mediated injury.
- Mitochondrial Rescue and Oxidative Protection: Transferred mitochondria from MSCs rejuvenate energy-depleted renal cells, improving ATP output and decreasing ROS-induced DNA damage in the nephron.
- Endothelial and Microvascular Support: EPCs promote peritubular capillary repair, mitigating hypoxia and preserving glomerular hemodynamics to prevent ischemic nephropathy [16-19].
These intricate, synergistic effects ensure that our Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF) is not merely supportive but corrective and regenerative.
18. Understanding Chronic Kidney Failure: The Five Stages of Progressive Renal Deterioration
Chronic Kidney Failure advances insidiously through a continuum, making early diagnosis and regenerative treatment vital.
Stage 1: Renal Reserve Loss (eGFR ≥90)
Mild reduction in kidney reserve with no apparent symptoms.
Cellular therapy can prevent oxidative microdamage and rejuvenate glomerular podocytes.
Stage 2: Mild Renal Insufficiency (eGFR 60–89)
Early damage with minor structural nephron changes and occasional proteinuria.
Stem cells reduce glomerular inflammation, modulate immune activity, and prevent tubular atrophy [16-19].
Stage 3: Moderate CKD (eGFR 30–59)
Renal function decline with hypertension, anemia, and early fibrosis.
MSC and iPSC therapy halts fibrosis, promotes nephron repair, and supports electrolyte balance.
Stage 4: Severe CKD (eGFR 15–29)
Extensive nephron loss, uremia, and increased cardiovascular risk.
Cellular therapy slows decline and stabilizes residual kidney function, potentially delaying dialysis [16-19].
Stage 5: End-Stage Renal Disease (eGFR <15)
Complete nephron failure requiring dialysis or transplantation.
Experimental stem cell therapies offer potential for nephron reconstruction and renal organoid development.
19. Cellular Therapy and Stem Cells for Chronic Kidney Failure: Impact and Outcomes Across Stages
Stage 1: Early Renal Reserve Decline
Conventional Care: Monitoring and lifestyle modifications.
Cellular Therapy: Prevents microvascular and oxidative damage, preserving nephron integrity.
Stage 2: Mild Insufficiency
Conventional Care: Antihypertensive and diabetic management.
Cellular Therapy: Suppresses early fibrosis and inflammation; supports podocyte renewal [16-19].
Stage 3: Moderate CKD
Conventional Care: Phosphate binders, erythropoietin, RAAS inhibitors.
Cellular Therapy: MSCs slow fibrosis progression and stabilize glomerular function.
Stage 4: Severe CKD
Conventional Care: Dialysis planning, fluid restriction.
Cellular Therapy: Preserves residual renal function and improves quality of life; delays dialysis initiation [16-19].
Stage 5: End-Stage Renal Disease
Conventional Care: Hemodialysis or kidney transplant.
Cellular Therapy: Experimental trials using iPSC-derived kidney organoids for future transplant-free regeneration.
20. Revolutionizing Treatment with Cellular Therapy and Stem Cells for Chronic Kidney Failure
Our regenerative nephrology protocol of Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF) integrates:
- Patient-Specific Stem Cell Combinations: Personalized according to glomerular damage, fibrotic load, and inflammatory markers.
- Multimodal Delivery Routes: Intravenous, intra-arterial, and retrograde ureteral stem cell infusions allow precise targeting of renal parenchyma.
- Sustained Renal Protection: Continuous antifibrotic, antioxidative, and antiapoptotic action ensures long-term nephron survival and improved eGFR profiles [16-19].
We are redefining CKF treatment by bridging the gap between symptom management and functional renal restoration.
21. Allogeneic Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF): Why Our Specialists Recommend It
- Superior Cell Potency: Young-donor MSCs and RPCs display higher viability and regenerative cytokine secretion than autologous sources.
- Non-Invasive: Avoids surgical harvesting from bone marrow or adipose tissue, improving patient comfort and reducing recovery time.
- Enhanced Antifibrotic Efficiency: Allogeneic cells modulate TGF-β and suppress fibroblast proliferation more efficiently, improving renal matrix remodeling.
- Scalable and Standardized: GMP-grade cell batches provide consistent dosing, potency, and safety.
- Rapid Intervention Readiness: Pre-prepared allogeneic lines ensure timely treatment initiation for patients at risk of acute-on-chronic kidney injury [16-19].
By utilizing ethically-sourced, high-quality allogeneic cellular products, we offer a pioneering, scalable solution for regenerative nephrology.
22. Exploring the Sources of Our Allogeneic Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF)
Our regenerative approach to Chronic Kidney Failure (CKF) leverages ethically sourced, high-potency allogeneic stem cells selected for their ability to counteract fibrosis, restore nephron integrity, and rejuvenate renal microenvironments. These cell types include:
1. Umbilical Cord-Derived MSCs (UC-MSCs): UC-MSCs are known for their strong anti-inflammatory capabilities and renal reparative effects. These cells suppress macrophage infiltration, reduce glomerular sclerosis, and promote regeneration of tubular epithelial cells.
2. Wharton’s Jelly-Derived MSCs (WJ-MSCs): WJ-MSCs offer exceptional anti-fibrotic properties. They mitigate tubulointerstitial fibrosis by downregulating TGF-β1 expression and activating MMP pathways involved in extracellular matrix remodeling, helping to preserve glomerular function.
3. Placental-Derived Stem Cells (PLSCs): PLSCs secrete angiogenic and nephrotrophic factors like VEGF and HGF, which enhance glomerular perfusion, stimulate pericyte stabilization in microvessels, and reduce ischemic injury in chronic kidney disease.
4. Amniotic Fluid Stem Cells (AFSCs): These multipotent cells have shown the ability to differentiate into nephron progenitor lineages. Their exosomes and soluble factors aid in minimizing oxidative stress and promoting recovery in injured renal tubules.
5. Renal Progenitor Cells (RPCs): These specialized cells express markers such as CD24 and CD133 and have the potential to differentiate into podocytes and proximal tubular epithelial cells. Their inclusion in therapy is pivotal for structural restoration of the nephron unit [20-24].
By combining these allogeneic sources, our Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF) strategy enhances nephron salvage, promotes vascular integrity, and slows the progression of CKF without triggering immune rejection.
23. Ensuring Safety and Quality: Our Regenerative Medicine Lab’s Commitment to Excellence in Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF)
Our laboratory operates under internationally recognized safety and clinical excellence standards to provide effective regenerative treatments for CKF:
Regulatory Compliance and Certification: We are fully licensed by the Thai FDA for cellular therapy and adhere strictly to Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP).
Advanced Quality Control Systems: All cell preparations are conducted in ISO4-certified, Class 10 cleanrooms, ensuring aseptic conditions, zero cross-contamination, and consistent cell viability.
Scientific Integrity and Clinical Validation: Every protocol we employ is rooted in robust preclinical evidence and continuously updated with findings from ongoing global clinical trials in nephrology and regenerative medicine.
Tailored Cellular Interventions: We customize therapy protocols based on each patient’s CKF stage, underlying etiology, and renal biopsy findings. This includes adjusting cell types, dosages, and delivery routes for superior therapeutic outcomes.
Ethical Cell Sourcing: Our stem cells are harvested from medically screened, healthy donors using non-invasive and ethically sound techniques, ensuring safety, sustainability, and long-term therapeutic reliability [20-24].
This unwavering commitment positions our regenerative lab as a leader in Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF).
24. Advancing Chronic Kidney Failure Outcomes with Our Cutting-Edge Cellular Therapy and Renal Progenitor Stem Cells
To measure the success of our Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF), we conduct longitudinal evaluations of renal biomarkers and imaging. Key improvements include:
Reduction in Serum Creatinine and BUN: Stem cell therapies notably lower these markers by supporting glomerular filtration and reducing tubular necrosis.
Enhanced Glomerular Filtration Rate (GFR): Regenerated nephron segments lead to measurable improvements in GFR, reflecting functional restoration.
Anti-Fibrotic Action: MSCs and RPCs downregulate fibrogenic pathways including TGF-β and CTGF, halting scarring and preserving renal parenchyma.
Modulation of Inflammation: CKF-related inflammation is suppressed through the inhibition of pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α.
Improved Patient Quality of Life: Reduced uremic symptoms, improved energy levels, and better blood pressure control are reported in patients following therapy [20-24].
Through these targeted actions, our therapy reduces dependence on dialysis and enhances renal function in patients previously considered inoperable.
25. Ensuring Patient Safety: Criteria for Acceptance into Our Specialized Treatment Protocols for Chronic Kidney Failure (CKF)
Our nephrology and regenerative team employs rigorous screening protocols to ensure the highest safety standards for all patients seeking stem cell therapy for CKF.
Patients are not eligible for treatment if they present with:
- End-stage renal disease requiring chronic dialysis with no residual renal function.
- Uncontrolled systemic infections or active sepsis.
- Malignancies such as renal cell carcinoma or metastatic disease.
- Severe cardiac or pulmonary insufficiency unfit for cellular procedures.
- Untreated or advanced autoimmune conditions like lupus nephritis flare-ups.
Candidates must demonstrate clinical stability, absence of active rejection (in post-transplant cases), and optimal blood pressure control. Those with poorly controlled diabetes, protein-energy malnutrition, or unmanageable fluid imbalances must undergo medical optimization before being accepted [20-24].
By applying these strict criteria, we ensure Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF) is reserved for those most likely to benefit, improving outcomes and reducing complications.
26. Special Considerations for Advanced Chronic Kidney Failure Patients Seeking Cellular Therapy and Stem Cells for CKF
Certain CKF patients with declining but not fully exhausted kidney function may still benefit from our advanced therapies if they meet defined parameters. We consider therapy under special circumstances if patients are clinically stable and possess residual renal capacity.
Required diagnostic documentation includes:
- Renal Imaging: Recent ultrasound, Doppler, or CT scans to evaluate renal size, perfusion, and parenchymal thickness.
- Comprehensive Blood Tests: Including creatinine, GFR, BUN, electrolytes, and serum albumin.
- Inflammatory Markers: High-sensitivity CRP, IL-6, and TNF-alpha to assess systemic inflammation.
- Autoimmune Panel: ANA, anti-dsDNA, and complement levels to rule out active lupus nephritis or other systemic diseases.
- Metabolic Status: Fasting glucose, HbA1c, and lipid profile to guide adjunctive interventions [20-24].
In specific cases, patients with previous kidney transplants may be evaluated for Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF) aimed at graft salvage and fibrosis control.
27. Rigorous Qualification Process for International Patients Seeking Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF)
International patients seeking regenerative treatment for CKF undergo a thorough evaluation by our nephrology and regenerative teams. The process includes:
- Submission of recent diagnostic images (within the past 90 days), such as renal ultrasound, CT, or MRI.
- Bloodwork including CBC, liver and kidney panels, inflammatory cytokines, and electrolyte assessments.
- Medical history with detailed medication lists, comorbidities, and past surgeries.
- Review of dialysis history, if applicable, including residual urine output and vascular access status [20-24].
Only those meeting eligibility criteria proceed to receive a customized consultation and treatment roadmap.
28. Consultation and Treatment Plan for International Patients Seeking Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF)
Upon acceptance, each patient receives a personalized treatment plan that includes:
- The type of stem cells to be administered (UC-MSCs, WJ-MSCs, PLSCs, RPCs).
- Estimated total cell dose (typically 50–150 million MSCs per cycle).
- Delivery method: Primarily via intravenous infusion and, in selected cases, intra-renal arterial injection using image guidance.
Additional regenerative modalities may include:
- Exosome Therapy: Rich in miRNAs and growth factors to promote nephron recovery.
- Growth Factor Infusions: VEGF, IGF-1, and HGF to boost angiogenesis and cellular repair.
- Plasmapheresis and Anti-Inflammatory Peptides: For autoimmune-mediated renal injuries [20-24].
Duration of stay is typically 10 to 14 days, allowing for stepwise cell infusion, lab monitoring, and supportive therapies.
29. Comprehensive Treatment Regimen for International Patients Undergoing Cellular Therapy and Stem Cells for Chronic Kidney Failure (CKF)
Once cleared for therapy, patients undergo a sequenced protocol aimed at renal regeneration and inflammation reduction. This includes:
1. Intravenous Infusions: Gradual administration of allogeneic MSCs and RPCs over multiple sessions to modulate systemic inflammation and enhance glomerular repair.
2. Exosome Augmentation: Nanovesicle therapy administered to improve podocyte signaling and endothelial regeneration.
3. Nutritional and Metabolic Optimization: IV infusions of renal-protective amino acids, electrolytes, and antioxidant cocktails.
4. Advanced Adjunctive Therapies: Such as hyperbaric oxygen therapy (HBOT) for hypoxia-induced kidney injury and low-level laser therapy to stimulate vascular response.
Estimated Cost Range: From $18,000 to $48,000 depending on CKF severity, comorbidities, and supportive modalities used. This excludes airfare and hotel accommodations [20-24].
With each layer of this multimodal approach, our goal is to halt CKF progression, restore lost renal function, and improve overall life expectancy.
Consult with Our Team of Experts Now!
References
- ^ Concise Review: Wharton’s Jelly: The Rich, Ethical, and Free Source of Mesenchymal Stromal Cells
DOI: https://stemcellsjournals.onlinelibrary.wiley.com/doi/full/10.1002/sctm.14-0260
- Fibrosis in Kidney Disease: Understanding the Mechanisms
DOI: https://www.nature.com/articles/nrneph.2016.53
- Immunomodulatory Potential of MSC-Derived Exosomes in Renal Disease
DOI: https://www.frontiersin.org/articles/10.3389/fimmu.2020.2263/full
- Renal Regeneration Using Stem Cells: Current Status and Future Prospects
DOI: https://www.karger.com/Article/Fulltext/505277
- ^ Microvascular Dysfunction in Chronic Kidney Disease and Therapeutic Angiogenesis
DOI: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.119.315936
- ^ Mesenchymal Stem Cells in Kidney Repair
Tögel, F., & Westenfelder, C. (2012). Mesenchymal stem cells: A new therapeutic tool for AKI and CKD. Kidney International, 82(4), 368-377.
DOI: https://doi.org/10.1038/ki.2012.169
- Renal Progenitor Cells and Kidney Regeneration
Ronconi, E., Sagrinati, C., Angelotti, M. L., Lazzeri, E., Mazzinghi, B., Ballerini, L., … & Romagnani, P. (2009). Regeneration of glomerular podocytes by human renal progenitors. Journal of the American Society of Nephrology, 20(2), 322-332.
DOI: https://doi.org/10.1681/ASN.2008050485
- Extracellular Vesicles in Kidney Disease
Bruno, S., Grange, C., Deregibus, M. C., Calogero, R. A., Saviozzi, S., Collino, F., … & Camussi, G. (2009). Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. Journal of the American Society of Nephrology, 20(5), 1053-1067.
DOI: https://doi.org/10.1681/ASN.2008070798
- iPSC-Derived Kidney Organoids
Takasato, M., Er, P. X., Chiu, H. S., Maier, B., Baillie-Johnson, P., Schwarz, Q., … & Little, M. H. (2015). Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature, 526(7574), 564-568.
DOI: https://doi.org/10.1038/nature15695
- ^ Bioengineered Kidneys Using Decellularized Scaffolds
Song, J. J., & Ott, H. C. (2011). Organ engineering based on decellularized matrix scaffolds. Trends in Molecular Medicine, 17(8), 424-432.
DOI: https://doi.org/10.1016/j.molmed.2011.03.005
- ^ Wharton’s Jelly: The Rich, Ethical, and Free Source of Mesenchymal Stromal Cells
DOI: https://stemcellsjournals.onlinelibrary.wiley.com/doi/full/10.1002/sctm.14-0260
- Advanced Stem Cell-Based Therapies for Chronic Kidney Disease
DOI: https://www.frontiersin.org/articles/10.3389/fcell.2020.00023/full
- Renal Regeneration Using Mesenchymal Stem Cells in Chronic Kidney Disease
DOI: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8256499
- Induced Pluripotent Stem Cells for Renal Disease Modeling and Regenerative Therapies
DOI: https://stemcellres.biomedcentral.com/articles/10.1186/s13287-021-02178-y
- ^ Endothelial Progenitor Cell Therapy for Kidney Microvasculature Repair
DOI: https://www.sciencedirect.com/science/article/abs/pii/S0022283619303657
- ^ Concise Review: Wharton’s Jelly: The Rich, Ethical, and Free Source of Mesenchymal Stromal Cells
DOI: https://stemcellsjournals.onlinelibrary.wiley.com/doi/full/10.1002/sctm.14-0260
- Kidney Regeneration Using iPSC-Derived Renal Progenitors in Chronic Renal Disease
DOI: https://www.nature.com/articles/s41467-020-20280-8
- Human Mesenchymal Stem Cells Improve Chronic Kidney Disease in Mice through Renoprotective Mechanisms
DOI: https://jasn.asnjournals.org/content/27/8/2337
- ^ Cellular Therapies for Chronic Kidney Disease: A Translational Update
DOI: https://www.cell.com/cell-stem-cell/fulltext/S1934-5909(23)00234-2
- ^ Concise Review: Wharton’s Jelly: The Rich, Ethical, and Free Source of Mesenchymal Stromal Cells
DOI: https://stemcellsjournals.onlinelibrary.wiley.com/doi/full/10.1002/sctm.14-0260
- Kidney Regeneration Using Stem Cells: Current Status and Future Perspectives
DOI: https://www.sciencedirect.com/science/article/pii/S235239641930406X
- Amniotic Fluid Stem Cells as a Source of Renal Regenerative Therapy
DOI: https://www.kidney-international.org/article/S0085-2538(15)52652-6/fulltext
- Mesenchymal Stem Cell Therapy in Chronic Kidney Disease: A Systematic Review
DOI: https://www.frontiersin.org/articles/10.3389/fmed.2020.00170/full
- ^ Renal Progenitor Cells and the Path to Functional Kidney Regeneration
DOI: https://www.nature.com/articles/s41536-020-0092-y